Neoadjuvant Therapy for Esophageal Adenocarcinoma in the Community Setting-Practice and Outcomes
- PMID: 28770168
- PMCID: PMC5513914
- DOI: 10.3389/fonc.2017.00151
Neoadjuvant Therapy for Esophageal Adenocarcinoma in the Community Setting-Practice and Outcomes
Retraction in
-
Retraction: Neoadjuvant Therapy for Esophageal Adenocarcinoma in the Community Setting-Practice and Outcomes.Front Oncol. 2019 Apr 24;9:324. doi: 10.3389/fonc.2019.00324. eCollection 2019. Front Oncol. 2019. PMID: 31106154 Free PMC article.
Abstract
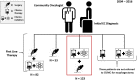
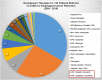
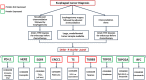
There has been an alarming rise in the incidence of esophageal adenocarcinoma which continues to have poor survival rates primarily due to lack of effective chemotherapy and presentation at advanced stages. Over a dozen chemotherapeutic agents are FDA approved for esophageal cancer (EC), and a two or three-drug combination is typically prescribed as first-line therapy for the majority of EC patients, administered either pre or post-operatively with esophageal resection. We have noticed significant variability in adjuvant and neoadjuvant regimens used in the community setting. The aim of this study was to review the various drug regimens used in the neoadjuvant setting for EC patients with adenocarcinoma undergoing resection at a single tertiary referral center in the Midwest. A total of 123 patients (stage II-III) underwent esophageal resection after neoadjuvant treatment at the center. Overall, 18 distinct drug regimens were used in 123 patients including two patients who received targeted therapy. Median survival post-surgery for this group was 11.2 months with no single regimen offering a survival advantage. These results reveal an unclear algorithm of how accepted regimens are prescribed in the community setting as well as a dire need for agents that are more effective. Additionally, it was noted that although proteomic markers have been found to predict drug response to 92% of the FDA-approved drugs in EC (12 of 13), according to pathology reports, molecular diagnostic testing was not used to direct treatment in this cohort. We therefore propose potential strategies to improve clinical outcomes including the use of a robust molecular oncology diagnostic panel and discuss the potential role for targeted chemotherapy and/or immunotherapy in the management of EC patients.
Keywords: clinical outcomes; esophageal adenocarcinoma; molecular diagnostics; patient management; proteomics; targeted chemotherapy; targeted therapy.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

