Understanding and preventing type 1 diabetes through the unique working model of TrialNet
- PMID: 28770323
- PMCID: PMC5838353
- DOI: 10.1007/s00125-017-4384-2
Understanding and preventing type 1 diabetes through the unique working model of TrialNet
Erratum in
-
Erratum to: Understanding and preventing type 1 diabetes through the unique working model of TrialNet.Diabetologia. 2017 Dec;60(12):2540. doi: 10.1007/s00125-017-4446-5. Diabetologia. 2017. PMID: 28948305 Free PMC article. No abstract available.
Abstract
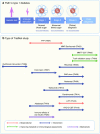
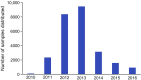
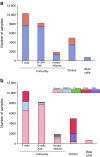
Type 1 diabetes is an autoimmune disease arising from the destruction of pancreatic insulin-producing beta cells. The disease represents a continuum, progressing sequentially at variable rates through identifiable stages prior to the onset of symptoms, through diagnosis and into the critical periods that follow, culminating in a variable depth of beta cell depletion. The ability to identify the very earliest of these presymptomatic stages has provided a setting in which prevention strategies can be trialled, as well as furnishing an unprecedented opportunity to study disease evolution, including intrinsic and extrinsic initiators and drivers. This niche opportunity is occupied by Type 1 Diabetes TrialNet, an international consortium of clinical trial centres that leads the field in intervention and prevention studies, accompanied by deep longitudinal bio-sampling. In this review, we focus on discoveries arising from this unique bioresource, comprising more than 70,000 samples, and outline the processes and science that have led to new biomarkers and mechanistic insights, as well as identifying new challenges and opportunities. We conclude that via integration of clinical trials and mechanistic studies, drawing in clinicians and scientists and developing partnership with industry, TrialNet embodies an enviable and unique working model for understanding a disease that to date has no cure and for designing new therapeutic approaches.
Keywords: Autoimmunity; Mechanistic studies; Review; Type 1 diabetes.
Conflict of interest statement
Duality of interest
MSA owns stocks of Medtronic. ÅL is a member of the Scientific Advisory Board of Diamyd Medical, Stockholm, Sweden. CJG is a consultant for Bristol-Myers Squibb and receives research funding from Janssen. All other authors declare that there is no duality of interest associated with their contributions to this manuscript.
Contribution statement
MB and MP drafted the article. All other authors revised it critically for important intellectual content. All authors approved the final version.
Figures

References
Publication types
MeSH terms
Grants and funding
- U01 DK085476/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- TrialNet/National Institute of Allergy and Infectious Diseases
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK061010, U01 DK061034, U01 DK061042, U01 DK06/DK/NIDDK NIH HHS/United States
- U01 DK103282/DK/NIDDK NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK085465/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK103180/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK103266/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

