High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya
- PMID: 28770596
- PMCID: PMC5577724
- DOI: 10.7448/IAS.20.5.21673
High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya
Abstract
Introduction: The 2015 WHO recommendation of antiretroviral therapy (ART) for all HIV-positive persons calls for treatment initiation in millions of persons newly eligible with high CD4+ counts. Efficient and effective care models are urgently needed for this population. We evaluated clinical outcomes of asymptomatic HIV-positive adults and children starting ART with high CD4+ counts using a novel streamlined care model in rural Uganda and Kenya.
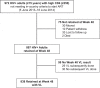
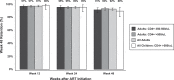
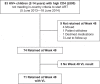
Methods: In the 16 intervention communities of the HIV test-and-treat Sustainable East Africa Research for Community Health Study (NCT01864603), all HIV-positive individuals irrespective of CD4 were offered ART (efavirenz [EFV]/tenofovir disoproxil fumarate + emtricitabine (FTC) or lamivudine (3TC). We studied adults (≥fifteen years) with CD4 ≥ 350/μL and children (two to fourteen years) with CD4 > 500/μL otherwise ineligible for ART by country guidelines. Clinics implemented a patient-centred streamlined care model designed to reduce patient-level barriers and maximize health system efficiency. It included (1) nurse-conducted visits with physician referral of complex cases, (2) multi-disease chronic care (including for hypertension/diabetes), (3) patient-centred, friendly staff, (4) viral load (VL) testing and counselling, (5) three-month return visits and ART refills, (6) appointment reminders, (7) tiered tracking for missed appointments, (8) flexible clinic hours (outside routine schedule) and (9) telephone access to clinicians. Primary outcomes were 48-week retention in care, viral suppression (% with measured week 48 VL ≤ 500 copies/mL) and adverse events. Results Overall, 972 HIV-positive adults with CD4+ ≥ 350/μL initiated ART with streamlined care. Patients were 66% female and had median age thirty-four years (IQR, 28-42), CD4+ 608/μL (IQR, 487-788/μL) and VL 6775 copies/mL (IQR, <500-37,003 c/mL). At week 48, retention was 92% (897/972; 2 died/40 moved/8 withdrew/4 transferred care/21/964 [2%] were lost to follow-up). Viral suppression occurred in 778/838 (93%) and 800/972 (82%) in intention-to-treat analysis. Grade III/IV clinical/laboratory adverse events were rare: 95 occurred in 74/972 patients (7.6%). Only 8/972 adults (0.8%) switched ART from EFV to lopinavir (LPV) (n = 2 for dizziness, n = 2 for gynaecomastia, n = 4 for other reasons). Among 83 children, week 48 retention was 89% (74/83), viral suppression was 92% (65/71) and grade III/IV adverse events occurred in 4/83 (4.8%).
Conclusions: Using a streamlined care model, viral suppression, retention and ART safety were high among asymptomatic East African adults and children with high CD4+ counts initiating treatment.
Clinical trial number: NCT01864603.
Keywords: .
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4+ T-cell counts above 350 cells/μl in rural Uganda.AIDS. 2014 Sep 24;28(15):2241-9. doi: 10.1097/QAD.0000000000000401. AIDS. 2014. PMID: 25022596 Free PMC article. Clinical Trial.
-
Estimated Costs for Delivery of HIV Antiretroviral Therapy to Individuals with CD4+ T-Cell Counts >350 cells/uL in Rural Uganda.PLoS One. 2015 Dec 3;10(12):e0143433. doi: 10.1371/journal.pone.0143433. eCollection 2015. PLoS One. 2015. PMID: 26632823 Free PMC article.
-
Improved Viral Suppression With Streamlined Care in the SEARCH Study.J Acquir Immune Defic Syndr. 2020 Dec 15;85(5):571-578. doi: 10.1097/QAI.0000000000002508. J Acquir Immune Defic Syndr. 2020. PMID: 32991337 Free PMC article.
-
Differentiated HIV care in sub-Saharan Africa: a scoping review to inform antiretroviral therapy provision for stable HIV-infected individuals in Kenya.AIDS Care. 2018 Dec;30(12):1477-1487. doi: 10.1080/09540121.2018.1500995. Epub 2018 Jul 23. AIDS Care. 2018. PMID: 30037312
-
Retention in care of HIV-infected children from HIV test to start of antiretroviral therapy: systematic review.PLoS One. 2013;8(2):e56446. doi: 10.1371/journal.pone.0056446. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437135 Free PMC article.
Cited by
-
Why do people living with HIV not initiate treatment? A systematic review of qualitative evidence from low- and middle-income countries.Soc Sci Med. 2018 Sep;213:72-84. doi: 10.1016/j.socscimed.2018.05.048. Epub 2018 May 30. Soc Sci Med. 2018. PMID: 30059900 Free PMC article.
-
Provider and Patient Perspectives of Rapid ART Initiation and Streamlined HIV Care: Qualitative Insights From Eastern African Communities.J Int Assoc Provid AIDS Care. 2021 Jan-Dec;20:23259582211053518. doi: 10.1177/23259582211053518. J Int Assoc Provid AIDS Care. 2021. PMID: 34841945 Free PMC article. Clinical Trial.
-
Population mobility associated with higher risk sexual behaviour in eastern African communities participating in a Universal Testing and Treatment trial.J Int AIDS Soc. 2018 Jul;21 Suppl 4(Suppl Suppl 4):e25115. doi: 10.1002/jia2.25115. J Int AIDS Soc. 2018. PMID: 30027668 Free PMC article.
-
Short Communication: Early Antiretroviral Therapy Is Associated with Better Viral Suppression and Less HIV Drug Resistance After Implementation of Universal Treatment in South Africa.AIDS Res Hum Retroviruses. 2020 Apr;36(4):297-299. doi: 10.1089/AID.2019.0206. Epub 2019 Dec 4. AIDS Res Hum Retroviruses. 2020. PMID: 31663368 Free PMC article. Clinical Trial.
-
"Hurdles on the path to 90-90-90 and beyond": Qualitative analysis of barriers to engagement in HIV care among individuals in rural East Africa in the context of test-and-treat.PLoS One. 2018 Aug 30;13(8):e0202990. doi: 10.1371/journal.pone.0202990. eCollection 2018. PLoS One. 2018. PMID: 30161172 Free PMC article. Clinical Trial.
References
-
- World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva, Switzerland: WHO; 2016. - PubMed
-
- Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. - PubMed
-
- Joint United Nations Programme on HIV/AIDS 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

