Paracetamol (acetaminophen) for chronic non-cancer pain in children and adolescents
- PMID: 28770975
- PMCID: PMC6484395
- DOI: 10.1002/14651858.CD012539.pub2
Paracetamol (acetaminophen) for chronic non-cancer pain in children and adolescents
Abstract
Background: Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for children's persisting pain acknowledge that pain in children is a major public health concern of high significance in most parts of the world. While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important.We designed a suite of seven reviews on chronic non-cancer pain and cancer pain (looking at antidepressants, antiepileptic drugs, non-steroidal anti-inflammatory drugs, opioids, and paracetamol as priority areas) in order to review the evidence for children's pain utilising pharmacological interventions in children and adolescents.As the leading cause of morbidity in children and adolescents in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain (lasting three months or longer) can arise in the paediatric population in a variety of pathophysiological classifications: nociceptive, neuropathic, idiopathic, visceral, nerve damage pain, chronic musculoskeletal pain, and chronic abdominal pain, and other unknown reasons.Paracetamol (acetaminophen) is one of the most widely used analgesics in both adults and children. The recommended dosage in the UK, Europe, Australia, and the USA for children and adolescents is generally 10 to 15 mg/kg every four to six hours, with specific age ranges from 60 mg (6 to 12 months old) up to 500 to 1000 mg (over 12 years old). Paracetamol is the only recommended analgesic for children under 3 months of age. Paracetamol has been proven to be safe in appropriate and controlled dosages, however potential adverse effects of paracetamol if overdosed or overused in children include liver and kidney failure.
Objectives: To assess the analgesic efficacy and adverse events of paracetamol (acetaminophen) used to treat chronic non-cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid, and Embase via Ovid from inception to 6 September 2016. We also searched the reference lists of retrieved studies and reviews, and searched online clinical trial registries.
Selection criteria: Randomised controlled trials, with or without blinding, of any dose and any route, treating chronic non-cancer pain in children and adolescents, comparing paracetamol with placebo or an active comparator.
Data collection and analysis: Two review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio and numbers needed to treat, using standard methods where data were available. We assessed GRADE (Grading of Recommendations Assessment, Development and Evaluation) and planned to create a 'Summary of findings' table.
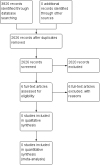
Main results: No studies were eligible for inclusion in this review. We rated the quality of the evidence as very low. We downgraded the quality of evidence by three levels due to the lack of data reported for any outcome.
Authors' conclusions: There was no evidence from randomised controlled trials to support or refute the use of paracetamol (acetaminophen) to treat chronic non-cancer pain in children and adolescents. We are unable to comment about efficacy or harm from the use of paracetamol to treat chronic non-cancer pain in children and adolescents.We know from adult randomised controlled trials that paracetamol, can be effective, in certain doses, and in certain pain conditions (not always chronic).This means that no conclusions could be made about efficacy or harm in the use of paracetamol (acetaminophen) to treat chronic non-cancer pain in children and adolescents.
Conflict of interest statement
CE: none known. Since CE is an author as well as the PaPaS Co‐ordinating Editor at the time of writing, we acknowledge the input of Neil O'Connell who acted as Sign Off Editor for this review. CE had no input into the editorial decisions or processes for this review.
TC: none known.
BA: none known; BA is a specialist anaesthetist and intensive care physician and manages the perioperative care of children requiring surgery and those critically ill requiring intensive care.
EF: none known.
NW: none known; NW is a specialist paediatric pain clinician and treats patients with chronic pain.
DGW: none known; DGW is a consultant in paediatric anaesthesia and pain medicine and treats children with acute and chronic pain.
Update of
References
References to studies excluded from this review
Ali 2007 {published data only}
-
- Ali Z, Burnett I, Eccles R, North M, Jawad M, Jawad S, et al. Efficacy of a paracetamol and caffeine combination in the treatment of the key symptoms of primary dysmenorrhoea. Current Medical Research and Opinion 2007;23(4):841‐51. - PubMed
Berry 1975 {published data only}
-
- Berry FN, Miller JM, Levin HM, Bare WW, Hopkinson JH 3rd, Feldman AJ. Relief of severe pain with acetaminophen in a new dose formulation versus propoxyphene hydrochloride 65 mg. and placebo: a comparative double‐blind study. Current Therapeutic Research, Clinical and Experimental 1975;17(4):361‐4. - PubMed
Cubero 2010 {published data only}
-
- Cubero DIG, Giglio A. Early switching from morphine to methadone is not improved by acetaminophen in the analgesia of concologic patients: a prospective, randomized, double‐blind, placebo‐controlled study. Support Care Cancer 2010;18:235‐42. - PubMed
McGuinness 1969 {published data only}
-
- McGuinness BW, Lloyd‐Jones M, Fowler PD. A double‐blind comparative trial of 'parazolidin' and paracetamol. British Journal of Clinical Practice 1969;23(11):452‐5. - PubMed
Mueller‐Lissner 2005 {published data only}
-
- Mueller‐Lissner S, Tytgat GN, Paulo LG, Guiqgleys EMM, Bubeck J, Peil H, et al. Placebo‐ and paracetamol‐controlled study on the efficacy and tolerability of hyoscine butylbromide in the treatment of patients with recurrent crampy abdominal pain. Alimentary Pharmacology & Therapies 2005;23:1741‐8. - PubMed
Valle‐Jones 1992 {published data only}
-
- Valle‐Jones JC, Walsh H, O'Hara J, O'Hara H, Davery NB, Hopkin‐Richards H. Controlled trial of a back support ('Lumbotrain') in patients with non‐specific low back pain. Current Medical Research and Opinion 1992;12(9):604‐13. - PubMed
Additional references
AMA 2013
-
- American Medical Association. Pediatric pain management. https://www.ama‐assn.org/ 2013 (accessed 25 January 2016).
Anderson 2015
-
- Anderson BJ, Hannam JA. Considerations when using PKPD modelling to determine effectiveness of simple analgesics in children. Expert Opinion on Drug Metabolism and Toxicology 2015;11(9):1393‐408. - PubMed
AUREF 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents 2012 (accessed 16 July 2016).
BNF 2016
-
- Joint Formulary Committee. British National Formulary. London (UK): BMJ Group and Pharmaceutical Press, 2016.
Caes 2016
Chan 2006
-
- Chan AT, Manson JE, Albert CM, Chae CU, Rexrode KM, Curhan GC, et al. Nonsteroidal anti inflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation 2006;113:1578‐87. - PubMed
Cooper 2017a
Cooper 2017b
Cooper 2017c
Daly 2008
-
- Daly FFS, Fountain JS, Murray L, Graudins A, Buckley NA. Guidelines for the management of paracetamol poisoning in Australia and New Zealand ‐ explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Medical Journal of Australia 2008;188:296‐301. - PubMed
Derry 2013
Dworkin 2008
Eccleston 2003
Eccleston 2017
FDA 2017
-
- US Food, Drug Administration. Acetaminophen information. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm (accessed 31 March 2017).
Forman 2005
-
- Forman JP, Stampfer MJ, Curhan GC. Non‐narcotic analgesic dose and risk of incident hypertension in US women. Hypertension 2005;46:500‐7. - PubMed
Forrest 1982
-
- Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clinical Pharmacokinetics 1982;7(2):93‐107. [PUBMED: 7039926] - PubMed
Graham 2013
-
- Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013;21:201‐32. - PubMed
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI: 10.1136/bmj.39489.470347.AD] - DOI - PMC - PubMed
Guyatt 2011
Guyatt 2013a
Guyatt 2013b
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hinz 2008
-
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase‐2 inhibitor in man. FASEB Journal 2008;22:383‐90. - PubMed
Hinz 2012
-
- Hinz B, Brune K. Paracetamol and cyclooxygenase inhibition: is there a cause for concern?. Annals of the Rheumatic Diseases 2012;71:20‐5. - PubMed
Hoffman 2010
Jozwiak‐Bebenista 2014
-
- Jozwiak‐Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Poloniae Pharmaceutica 2014;71:11‐23. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Machado 2015
Marjoribanks 2015
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford (UK): Oxford University Press, 1998.
Moher 2009
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle (WA): IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
Moore 2010b
-
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. - PubMed
Moore 2010c
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010d
Moore 2010e
-
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149:173‐6. [PUBMED: 19748185] - PubMed
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2011b
Moore 2012
Moore 2013a
Moore 2013b
Moore 2014a
-
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. - PubMed
Moore 2014b
Moore 2015
Moore 2016
NICE 2016
-
- National Institute for Health and Care Excellence. Analgesia ‐ mild‐to‐moderate pain. cks.nice.org.uk/analgesia‐mild‐to‐moderate‐pain#!scenario:1 2016 (accessed prior to 10 June 2017).
O'Brien 2010
Ohlsson 2016
PedIMMPACT 2008
-
- McGrath PJ, Walco GA, Turk DC, Dworking RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT. Journal of Pain 2008;9(9):771‐83. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 2008
Roberts 2016
Saragiotto 2016
Sheen 2002
-
- Sheen CL, Dillon JF, Bateman DN, Simpson KJ, Macdonald TM. Paracetamol toxicity: epidemiology, prevention and costs to the health‐care system. QJM: An International Journal of Medicine 2002;95:609‐19. - PubMed
Shinoda 2007
-
- Shinoda S, Aoyama T, Aoyama Y, Tomioka S, Matsumoto Y, Ohe Y. Pharamcokinetics/pharmacodynamics of acetaminophen analgesia in Japanese patients with chronic pain. Biological and Pharmaceutical Bulletin 2007;30(1):157‐61. - PubMed
Sjoukes 2016
-
- Sjoukes A, Venekamp RP, Pol AC, Hay AD, Little P, Schilder AGM, et al. Paracetamol (acetaminophen) or non‐steroidal anti‐inflammatory drugs, alone or combined, for pain relief in acute otitis media in children. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD011534.pub2] - DOI - PMC - PubMed
Stinson 2006
-
- Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self‐report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1‐2):143‐57. [DOI: 10.1016/j.pain.2006.05.006] - DOI - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
TGA 2017
-
- Therapeutic Goods Administration. Reccomended paracetamol doses. www.tga.gov.au/community‐qa/recommended‐paracetamol‐doses 2017 (accessed 31 March 2017).
United Nations 2015
-
- United Nations. World population prospects 2015 ‐ population indicators. esa.un.org/unpd/wpp/Download/Standard/Population/ 2015 (accessed 29 February 2016).
von Baeyer 2007
WHO 2012
-
- World Health Organization. WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. WHO Press, World Health Organization 2012. [ISBN 978 92 4 154812 0] - PubMed
Wiffen 2016
Wiffen 2017a
Wiffen 2017b
Wong 2013
World Bank 2014
-
- World Bank. Data ‐ population ages 0‐14 (% of total). data.worldbank.org/indicator/SP.POP.0014.TO.ZS 2014 (accessed 29 February 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous