HLA-DQB1*06 and breadth of Nef core region-specific T-cell response are associated with slow disease progression in antiretroviral therapy-naive Chinese HIV-1 subtype B patients
- PMID: 28771107
- PMCID: PMC5647954
- DOI: 10.1080/21645515.2017.1340138
HLA-DQB1*06 and breadth of Nef core region-specific T-cell response are associated with slow disease progression in antiretroviral therapy-naive Chinese HIV-1 subtype B patients
Abstract
Vaccines still are an important way to prevent and treat acquired immunodeficiency syndrome (AIDS). 1 For developing an effective T cell-based AIDS vaccine, it is critical to define the human leukocyte antigen (HLA) type and epitope that elicit the most potent responses. This study involved 29 antiretroviral therapy-naive and chronic human immunodeficiency virus (HIV)-1 subtype B-infected individuals. A polymerase chain reaction-sequence-specific primer was used to detect the HLA typing, and the enzyme-linked immunospot assay to quantify the T-cell immune function. The results showed that the HLA-DQB1*06-positive group had higher CD4 counts and lower viral load (VL) compared with the HLA-DQB1*06-negative group; A higher magnitude of HIV-1-specific T-cell response and breadth were observed in the HLA-DQB1*06-positive group; the T-cell response was proportional to VL (R2 = 0.488, P = 0.0368) in the HLA-DQB1*06-positive group. The total T-cell responses to HIV-1 Nef core region were quantified at the single-peptide level. Nine (90%) peptides were recognized in 18 (62.1%) individuals. The breath of Nef core region-specific T-cell response was correlated positively with CD4+ T cell count and inversely with VL, which improved disease outcomes. These data revealed that HLA-DQB1*06 had a protective effect on the course of HIV-1 and T-cell targeting of certain specific Nef epitopes, contributing to HIV-1 suppression. The results suggested the potential use of HLA-DQB1*06 and Nef core region in HIV-1 T-cell vaccine design.
Keywords: HIV-1; HLA; NEF T-cell response; Vaccine.
Figures





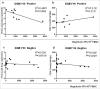
References
-
- Cohen KW, Frahm N. Current views on the potential for development of a HIV vaccine. Expert Opin Biol Ther 2017; 17:295-303; PMID:28095712; https://doi.org/ 10.1080/14712598.2017.1282457 - DOI - PMC - PubMed
-
- Cotton LA, Kuang XT, Le AQ, Carlson JM, Chan B, Chopera DR, Brumme CJ, Markle TJ, Martin E, Shahid A, et al.. Genotypic and functional impact of HIV-1 adaptation to its host population during the North American epidemic. Plos Genet 2014; 10:e1004295; PMID:24762668; https://doi.org/ 10.1371/journal.pgen.1004295 - DOI - PMC - PubMed
-
- Payne R, Muenchhoff M, Mann J, Roberts HE, Matthews P, Adland E, Hempenstall A, Huang KH, Brockman M, Brumme Z, et al.. Impact of HLA-driven HIV adaptation on virulence in populations of high HIV seroprevalence. Proc Natl Acad Sci U S A 2014; 111:E5393-400; PMID:25453107; https://doi.org/ 10.1073/pnas.1413339111 - DOI - PMC - PubMed
-
- Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity 2012; 37:426-40; PMID:22999948; https://doi.org/ 10.1016/j.immuni.2012.09.005 - DOI - PMC - PubMed
-
- Adland E, Paioni P, Thobakgale C, Laker L, Mori L, Muenchhoff M, Csala A, Clapson M, Flynn J, Novelli V, et al.. Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection. Plos Pathog 2015; 11:e1004954; PMID:26076345; https://doi.org/ 10.1371/journal.ppat.1004954 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
