Somatic Host Cell Alterations in HPV Carcinogenesis
- PMID: 28771191
- PMCID: PMC5580463
- DOI: 10.3390/v9080206
Somatic Host Cell Alterations in HPV Carcinogenesis
Abstract
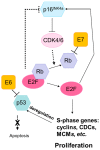
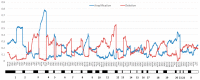
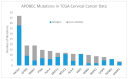
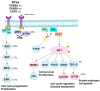
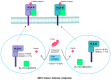
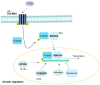
High-risk human papilloma virus (HPV) infections cause cancers in different organ sites, most commonly cervical and head and neck cancers. While carcinogenesis is initiated by two viral oncoproteins, E6 and E7, increasing evidence shows the importance of specific somatic events in host cells for malignant transformation. HPV-driven cancers share characteristic somatic changes, including apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC)-driven mutations and genomic instability leading to copy number variations and large chromosomal rearrangements. HPV-associated cancers have recurrent somatic mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and phosphatase and tensin homolog (PTEN), human leukocyte antigen A and B (HLA-A and HLA-B)-A/B, and the transforming growth factor beta (TGFβ) pathway, and rarely have mutations in the tumor protein p53 (TP53) and RB transcriptional corepressor 1 (RB1) tumor suppressor genes. There are some variations by tumor site, such as NOTCH1 mutations which are primarily found in head and neck cancers. Understanding the somatic events following HPV infection and persistence can aid the development of early detection biomarkers, particularly when mutations in precancers are characterized. Somatic mutations may also influence prognosis and treatment decisions.
Keywords: APOBEC; HPV; cervical cancer; chromosomal instability; copy number variation; head and neck cancer; integration; significantly mutated gene; somatic mutation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus.J Natl Cancer Inst. 2004 Jul 7;96(13):998-1006. doi: 10.1093/jnci/djh183. J Natl Cancer Inst. 2004. PMID: 15240783
-
The molecular mechanism of human papillomavirus-induced carcinogenesis in head and neck squamous cell carcinoma.Int J Clin Oncol. 2016 Oct;21(5):819-826. doi: 10.1007/s10147-016-1005-x. Epub 2016 Jun 23. Int J Clin Oncol. 2016. PMID: 27339270 Review.
-
APOBEC-mediated genomic alterations link immunity and viral infection during human papillomavirus-driven cervical carcinogenesis.Biosci Trends. 2017 Sep 12;11(4):383-388. doi: 10.5582/bst.2017.01103. Epub 2017 Jul 17. Biosci Trends. 2017. PMID: 28717061 Review.
-
The head and neck cancer cell oncogenome: a platform for the development of precision molecular therapies.Oncotarget. 2014 Oct 15;5(19):8906-23. doi: 10.18632/oncotarget.2417. Oncotarget. 2014. PMID: 25275298 Free PMC article.
-
Suppression of tumorigenesis by transcription units expressing the antisense E6 and E7 messenger RNA (mRNA) for the transforming proteins of the human papilloma virus and the sense mRNA for the retinoblastoma gene in cervical carcinoma cells.Cancer Gene Ther. 1995 Mar;2(1):19-32. Cancer Gene Ther. 1995. PMID: 7621252
Cited by
-
The Role of Human Papilloma Virus in Dictating Outcomes in Head and Neck Squamous Cell Carcinoma.Front Mol Biosci. 2021 Jun 23;8:677900. doi: 10.3389/fmolb.2021.677900. eCollection 2021. Front Mol Biosci. 2021. PMID: 34250016 Free PMC article. Review.
-
Next-Generation Sequencing-Based Molecular Profiling of Conjunctival Squamous Cell Carcinoma and Its Potential Application for Therapy.Ophthalmol Sci. 2025 Apr 23;5(5):100801. doi: 10.1016/j.xops.2025.100801. eCollection 2025 Sep-Oct. Ophthalmol Sci. 2025. PMID: 40529102 Free PMC article.
-
Genotypic distribution of human papillomavirus and phylogenetic analysis of E6 and E7 gene of HR-HPV variants isolated from Pakistani population.Medicine (Baltimore). 2023 Jan 13;102(2):e32651. doi: 10.1097/MD.0000000000032651. Medicine (Baltimore). 2023. PMID: 36637937 Free PMC article.
-
Development of a Novel Mouse Model of Spontaneous High-Risk HPVE6/E7-Expressing Carcinoma in the Cervicovaginal Tract.Cancer Res. 2021 Sep 1;81(17):4560-4569. doi: 10.1158/0008-5472.CAN-21-0399. Epub 2021 Jul 2. Cancer Res. 2021. PMID: 34215618 Free PMC article.
-
HPV-driven cancer: from epidemiology to the HPV-driven tumor board proposal, everything you wanted to know but were afraid to ask.Clin Transl Oncol. 2025 Aug;27(8):3511-3529. doi: 10.1007/s12094-025-03868-3. Epub 2025 Mar 6. Clin Transl Oncol. 2025. PMID: 40048019 Review.
References
-
- Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J., Peto J., Meijer C.J., Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
-
- Ndiaye C., Mena M., Alemany L., Arbyn M., Castellsague X., Laporte L., Bosch F.X., de Sanjose S., Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4A detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. - DOI - PubMed
-
- Hartwig S., Baldauf J.-J., Dominiak-Felden G., Simondon F., Alemany L., de Sanjosé S., Castellsagué X. Estimation of the epidemiological burden of HPV-related anogenital cancers, precancerous lesions, and genital warts in women and men in europe: Potential additional benefit of a nine-valent second generation HPV vaccine compared to first generation HPV vaccines. Papillomavirus Res. 2015;1:90–100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

