Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model
- PMID: 28771195
- PMCID: PMC5578088
- DOI: 10.3390/ijms18081698
Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model
Abstract
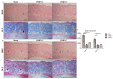
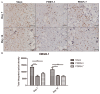
High-mobility group box protein-1 (HMGB-1) plays a central role in the inflammatory network, and uncontrolled chronic inflammation can lead to excessive scarring. The aim of this study was to evaluate the anti-inflammatory effects of polydeoxyribonucleotide (PDRN) on scar formation. Sprague-Dawley rats (n = 30) underwent dorsal excision of the skin, followed by skin repair. PDRN (8 mg/kg) was administered via intraperitoneal injection for three (PDRN-3 group, n = 8) or seven (PDRN-7 group, n = 8) days, and HMGB-1 was administered via intradermal injection in addition to PDRN treatment for three days (PDRN-3+HMGB-1 group; n = 6). The scar-reducing effects of PDRN were evaluated in the internal scar area and by inflammatory cell counts using histology and immunohistochemistry. Western blot, immunohistochemistry and immunofluorescence assays were performed to observe changes in type I and type III collagen and the expression of HMGB-1 and CD45. Treatment with PDRN significantly reduced the scar area, inflammatory cell infiltration and the number of CD45-positive cells. In addition, the increased expression of HMGB-1 observed in the sham group was significantly reduced after treatment with PDRN. Rats administered HMGB-1 in addition to PDRN exhibited scar areas with inflammatory cell infiltration similar to the sham group, and the collagen synthesis effects of PDRN were reversed. In summary, PDRN exerts anti-inflammatory and collagen synthesis effects via HMGB-1 suppression, preventing scar formation. Thus, we believe that the anti-inflammatory and collagen synthesis effects of PDRN resulted in faster wound healing and decreased scar formation.
Keywords: cicatrix, inflammation; polydeoxyribonucleotide; rats; wounds and injuries.
Conflict of interest statement
None of the authors have any financial arrangements or potential conflicts of interest related to this article.
Figures





Similar articles
-
Interleukin-10 reduces scar formation in both animal and human cutaneous wounds: results of two preclinical and phase II randomized control studies.Wound Repair Regen. 2013 May-Jun;21(3):428-36. doi: 10.1111/wrr.12043. Epub 2013 Apr 29. Wound Repair Regen. 2013. PMID: 23627460 Clinical Trial.
-
Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps.J Vasc Surg. 2012 Feb;55(2):479-88. doi: 10.1016/j.jvs.2011.07.083. Epub 2011 Nov 3. J Vasc Surg. 2012. PMID: 22051873
-
Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A(₂A) receptor.Arthritis Rheum. 2011 Nov;63(11):3364-71. doi: 10.1002/art.30538. Arthritis Rheum. 2011. PMID: 21769841
-
Scar-free healing: from embryonic mechanisms to adult therapeutic intervention.Philos Trans R Soc Lond B Biol Sci. 2004 May 29;359(1445):839-50. doi: 10.1098/rstb.2004.1475. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15293811 Free PMC article. Review.
-
Polydeoxyribonucleotide: A Promising Biological Platform to Accelerate Impaired Skin Wound Healing.Pharmaceuticals (Basel). 2021 Oct 29;14(11):1103. doi: 10.3390/ph14111103. Pharmaceuticals (Basel). 2021. PMID: 34832885 Free PMC article. Review.
Cited by
-
A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2.Molecules. 2022 Feb 14;27(4):1276. doi: 10.3390/molecules27041276. Molecules. 2022. PMID: 35209068 Free PMC article.
-
EW-7197, transforming growth factor β inhibitor, combined with irreversible electroporation for improving skin wound in a rat excisional model.Sci Rep. 2024 Jun 4;14(1):12779. doi: 10.1038/s41598-024-61003-8. Sci Rep. 2024. PMID: 38834729 Free PMC article.
-
Bone regeneration in ceramic scaffolds with variable concentrations of PDRN and rhBMP-2.Sci Rep. 2021 Jun 1;11(1):11470. doi: 10.1038/s41598-021-91147-w. Sci Rep. 2021. PMID: 34075179 Free PMC article.
-
Polydeoxyribonucleotide ameliorates IL-1β-induced impairment of chondrogenic differentiation in human bone marrow-derived mesenchymal stem cells.Sci Rep. 2024 Oct 30;14(1):26076. doi: 10.1038/s41598-024-77264-2. Sci Rep. 2024. PMID: 39478005 Free PMC article.
-
Polydeoxyribonucleotides Improve Diabetic Wound Healing in Mouse Animal Model for Experimental Validation.Ann Dermatol. 2019 Aug;31(4):403-413. doi: 10.5021/ad.2019.31.4.403. Epub 2019 Jul 1. Ann Dermatol. 2019. PMID: 33911618 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

