Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest
- PMID: 28771209
- PMCID: PMC5618036
- DOI: 10.3390/bios7030030
Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest
Abstract
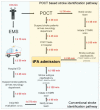
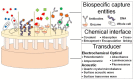
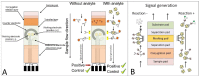
Stroke, the second highest leading cause of death, is caused by an abrupt interruption of blood to the brain. Supply of blood needs to be promptly restored to salvage brain tissues from irreversible neuronal death. Existing assessment of stroke patients is based largely on detailed clinical evaluation that is complemented by neuroimaging methods. However, emerging data point to the potential use of blood-derived biomarkers in aiding clinical decision-making especially in the diagnosis of ischemic stroke, triaging patients for acute reperfusion therapies, and in informing stroke mechanisms and prognosis. The demand for newer techniques to deliver individualized information on-site for incorporation into a time-sensitive work-flow has become greater. In this review, we examine the roles of a portable and easy to use point-of-care-test (POCT) in shortening the time-to-treatment, classifying stroke subtypes and improving patient's outcome. We first examine the conventional stroke management workflow, then highlight situations where a bedside biomarker assessment might aid clinical decision-making. A novel stroke POCT approach is presented, which combines the use of quantitative and multiplex POCT platforms for the detection of specific stroke biomarkers, as well as data-mining tools to drive analytical processes. Further work is needed in the development of POCTs to fulfill an unmet need in acute stroke management.
Keywords: Biomarkers; Data-Mining; Diagnostics; Multiplex and Quantitative Detection; Point-of-Care-Test; Stroke; Time-Dependent Treatment.
Conflict of interest statement
The authors declare no conflict of interest
Figures
References
-
- Bhavna J. Stroke Diagnostics and Therapeutics: Global Markets. BCC research; Wellesley, MA, USA: 2015.
-
- Goldstein L.B., Adams R., Alberts M.J., Appel L.J., Brass L.M., Bushnell C.D., Culebras A., Degraba T.J., Gorelick P.B., Guyton J.R., et al. Primary prevention of ischemic stroke: A guideline from the american heart association/american stroke association stroke council: Cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group: The American academy of neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. - PubMed
-
- Allender S., Scarborough P., Peto V., Rayner M., Leal J., Luengo-Fernandez R., Gray A. European Cardiovascular Disease Statistics. European Heart Network; Brussels, UK: 2008.
-
- Charles P.W., Jan V.G., Martin S.D., Joanna M.W., John M.B., Graeme J.H., Peter A.G.S., Gabriel R., Peter L., Cathie S., et al. Stroke: Practical Management. 3rd ed. Wiley-Blackwell; Tokyo, Japan: 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

