The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF
- PMID: 28771225
- PMCID: PMC5596551
- DOI: 10.1038/cddis.2017.361
The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF
Abstract
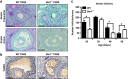
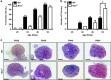
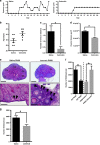
In females, germ cells are maintained in ovarian structures called primordial follicles. The number of primordial follicles in the ovarian reserve is a critical determinant of the length of the fertile lifespan. Despite this significance, knowledge of the precise physiological mechanisms that regulate primordial follicle number is lacking. In this study we show that a wave of primordial follicle depletion occurs during the transition to adulthood in mice. We demonstrate that this sudden and dramatic loss of primordial follicles is hormonally triggered and identify the pro-apoptotic BH3-only protein, BCL-2 modifying factor (BMF), as essential for this process, implicating the intrinsic apoptotic pathway as a key mechanism. The elimination of primordial follicles during puberty is not only a striking developmental event, it is also physiologically important because it ultimately reduces the availability of primordial follicles and determines the duration of fertility. Collectively, these findings show that puberty is a critical developmental window for the regulation of the size of ovarian reserve, impacting on female fertility and reproductive longevity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice.Biol Reprod. 2014 Apr 10;90(4):77. doi: 10.1095/biolreprod.113.116947. Print 2014 Apr. Biol Reprod. 2014. PMID: 24571986
-
The impact of passive immunisation against BMPRIB and BMP4 on follicle development and ovulation in mice.Reproduction. 2015 May;149(5):403-11. doi: 10.1530/REP-14-0451. Epub 2015 Feb 9. Reproduction. 2015. PMID: 25667430
-
Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions.Hum Mol Genet. 2014 Feb 15;23(4):920-8. doi: 10.1093/hmg/ddt486. Epub 2013 Oct 1. Hum Mol Genet. 2014. PMID: 24087793 Free PMC article.
-
Taking control of the female fertile lifespan: a key role for Bcl-2 family proteins.Reprod Fertil Dev. 2016 Jun;28(7):864-871. doi: 10.1071/RD14326. Reprod Fertil Dev. 2016. PMID: 25423414 Review.
-
How Is the Number of Primordial Follicles in the Ovarian Reserve Established?Biol Reprod. 2015 Nov;93(5):111. doi: 10.1095/biolreprod.115.133652. Epub 2015 Sep 30. Biol Reprod. 2015. PMID: 26423124 Review.
Cited by
-
Different cell death types determination in juvenile mice ovarian follicles.Iran J Vet Res. 2018 Fall;19(4):298-303. Iran J Vet Res. 2018. PMID: 30774671 Free PMC article.
-
Oocyte CTR1 is not essential for cisplatin-induced oocyte death of primordial follicle.MicroPubl Biol. 2022 Sep 1;2022:10.17912/micropub.biology.000632. doi: 10.17912/micropub.biology.000632. eCollection 2022. MicroPubl Biol. 2022. PMID: 36120475 Free PMC article.
-
Acylated Ghrelin Supports the Ovarian Transcriptome and Follicles in the Mouse: Implications for Fertility.Front Endocrinol (Lausanne). 2019 Jan 15;9:815. doi: 10.3389/fendo.2018.00815. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30697193 Free PMC article.
-
Senolytic treatment fails to improve ovarian reserve or fertility in female mice.Geroscience. 2024 Jun;46(3):3445-3455. doi: 10.1007/s11357-024-01089-0. Epub 2024 Feb 15. Geroscience. 2024. PMID: 38358579 Free PMC article.
-
Synergistic enhancement of the mouse Pramex1 and Pramel1 in repressing retinoic acid (RA) signaling during gametogenesis.Cell Biosci. 2024 Feb 23;14(1):28. doi: 10.1186/s13578-024-01212-w. Cell Biosci. 2024. PMID: 38395975 Free PMC article.
References
-
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21: 200–214. - PubMed
-
- Liew SH, Vaithiyanathan K, Cook M, Bouillet P, Scott CL, Kerr JB et al. Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice. Biol Reprod 2014; 90: 77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

