High-Content Assay Multiplexing for Vascular Toxicity Screening in Induced Pluripotent Stem Cell-Derived Endothelial Cells and Human Umbilical Vein Endothelial Cells
- PMID: 28771372
- PMCID: PMC5576216
- DOI: 10.1089/adt.2017.786
High-Content Assay Multiplexing for Vascular Toxicity Screening in Induced Pluripotent Stem Cell-Derived Endothelial Cells and Human Umbilical Vein Endothelial Cells
Abstract
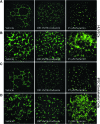
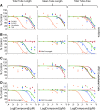
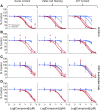
Endothelial cells (ECs) play a major role in blood vessel formation and function. While there is longstanding evidence for the potential of chemical exposures to adversely affect EC function and vascular development, the hazard potential of chemicals with respect to vascular effects is not routinely evaluated in safety assessments. Induced pluripotent stem cell (iPSC)-derived ECs promise to provide a physiologically relevant, organotypic culture model that is amenable for high-throughput (HT) EC toxicant screening and may represent a viable alternative to traditional in vitro models, including human umbilical vein endothelial cells (HUVECs). To evaluate the utility of iPSC-ECs for multidimensional HT toxicity profiling of chemicals, both iPSC-ECs and HUVECs were exposed to selected positive (angiogenesis inhibitors, cytotoxic agents) and negative compounds in concentration response for either 16 or 24 h in a 384-well plate format. Furthermore, chemical effects on vascularization were quantified using EC angiogenesis on biological (Geltrex™) and synthetic (SP-105 angiogenesis hydrogel) extracellular matrices. Cellular toxicity was assessed using high-content live cell imaging and the CellTiter-Glo® assay. Assay performance indicated good to excellent assay sensitivity and reproducibility for both cell types investigated. Both iPSC-derived ECs and HUVECs formed tube-like structures on Geltrex™ and hydrogel, an effect that was inhibited by angiogenesis inhibitors and cytotoxic agents in a concentration-dependent manner. The quality of HT assays in HUVECs was generally higher than that in iPSC-ECs. Altogether, this study demonstrates the capability of ECs for comprehensive assessment of the biological effects of chemicals on vasculature in a HT compatible format.
Keywords: angiogenesis; endothelial cells; high-throughput; iPSC-derived cells.
Conflict of interest statement
C.S.L. was employed by StemPharm, Incorporated, the manufacturer of the hydrogel used in this study.
Figures

Similar articles
-
Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays.Acta Biomater. 2016 Jul 15;39:12-24. doi: 10.1016/j.actbio.2016.05.020. Epub 2016 May 13. Acta Biomater. 2016. PMID: 27181878 Free PMC article.
-
High-Content Assay Multiplexing for Toxicity Screening in Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Hepatocytes.Assay Drug Dev Technol. 2015 Nov;13(9):529-46. doi: 10.1089/adt.2015.659. Epub 2015 Nov 5. Assay Drug Dev Technol. 2015. PMID: 26539751 Free PMC article.
-
Robust and Scalable Angiogenesis Assay of Perfused 3D Human iPSC-Derived Endothelium for Anti-Angiogenic Drug Screening.Int J Mol Sci. 2020 Jul 7;21(13):4804. doi: 10.3390/ijms21134804. Int J Mol Sci. 2020. PMID: 32645937 Free PMC article.
-
The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review.J Appl Toxicol. 2017 Dec;37(12):1359-1369. doi: 10.1002/jat.3470. Epub 2017 Apr 6. J Appl Toxicol. 2017. PMID: 28383141 Review.
-
Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models.Mol Cell Neurosci. 2016 Jun;73:104-15. doi: 10.1016/j.mcn.2016.01.011. Epub 2016 Jan 28. Mol Cell Neurosci. 2016. PMID: 26826498 Free PMC article. Review.
Cited by
-
Cardiovascular Effects of Polychlorinated Biphenyls and Their Major Metabolites.Environ Health Perspect. 2020 Jul;128(7):77008. doi: 10.1289/EHP7030. Epub 2020 Jul 23. Environ Health Perspect. 2020. PMID: 32701041 Free PMC article.
-
High-Content Assay Multiplexing for Muscle Toxicity Screening in Human-Induced Pluripotent Stem Cell-Derived Skeletal Myoblasts.Assay Drug Dev Technol. 2018 Aug/Sep;16(6):333-342. doi: 10.1089/adt.2018.860. Epub 2018 Aug 2. Assay Drug Dev Technol. 2018. PMID: 30070899 Free PMC article.
-
Potential Human Health Hazard of Post-Hurricane Harvey Sediments in Galveston Bay and Houston Ship Channel: A Case Study of Using In Vitro Bioactivity Data to Inform Risk Management Decisions.Int J Environ Res Public Health. 2021 Dec 19;18(24):13378. doi: 10.3390/ijerph182413378. Int J Environ Res Public Health. 2021. PMID: 34948986 Free PMC article.
-
Hazard and risk characterization of 56 structurally diverse PFAS using a targeted battery of broad coverage assays using six human cell types.Toxicology. 2024 Mar;503:153763. doi: 10.1016/j.tox.2024.153763. Epub 2024 Feb 27. Toxicology. 2024. PMID: 38423244 Free PMC article.
-
Neurovascular Organotypic Culture Models Using Induced Pluripotent Stem Cells to Assess Adverse Chemical Exposure Outcomes.Appl In Vitro Toxicol. 2019 Jun 1;5(2):92-110. doi: 10.1089/aivt.2018.0025. Epub 2019 Jun 17. Appl In Vitro Toxicol. 2019. PMID: 32292797 Free PMC article.
References
-
- Pruss-Ustun A, Corvalan C: Preventing Disease Through Healthy Environments: Towards an Estimate of the Environmental Burden of Disease. World Health Organization, Geneva, Switzerland, 2006
-
- Eckers A, Haendeler J: Endothelial cells in health and disease. Antioxid Redox Signal 2015;22:1209–1211 - PubMed
-
- Smith EJ, Staton CA: Tubule Formation Assays. John Wiley & Sons, Ltd., Hoboken, NJ, 2006
-
- Jennings P: The future of in vitro toxicology. Toxicol In Vitro 2015;29:1217–1221 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

