Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression?
- PMID: 28771573
- PMCID: PMC5542660
- DOI: 10.1371/journal.pone.0182427
Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression?
Abstract
Background: Sepsis is defined as a dysregulated immune response to infection. Impaired immune response in sepsis, often described as endotoxin tolerance, is characterized by unresponsiveness of monocytes on lipopolysaccharide (LPS) stimulation to release tumor necrosis factor α (TNFα). Furthermore, decreased monocyte surface protein expression of human leucocyte antigen DR (HLA-DR) is a marker for changes of the innate immune response during sepsis. Quantitative polymerase chain reaction (qPCR) and flow-cytometry (FACS) have been used to measure protein or gene expression of HLA-DR. We aimed to determine whether changes in mRNA expression of HLA-DR are associated with impaired TNFα response in human sepsis.
Methods: Surface protein together with mRNA expression of HLA-DR were measured by FACS and qPCR in a cohort of 9 sepsis patients and compared to 10 pre-operative control patients in a prospective study. In addition, 20 patients with post-surgical inflammation, 20 patients with sepsis or septic shock were included and TNFα was determined following ex vivo stimulation of whole blood with 500 pg/mL LPS. Total RNA was prepared from whole blood and subjected to qPCR analysis for expression analysis of HLA-DR alpha (HLA-DRA) to correlate TNFα response with HLA-DRA expression.
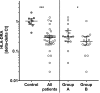
Results: Patients with sepsis presented higher numbers of monocytes in peripheral blood (P<0.001) but decreased surface protein and mRNA HLA-DR levels when compared to controls. In all patients mRNA expression of HLA-DRA was decreased by approximately 70% compared to controls (P<0.01) and was lowest in patients with sepsis or septic shock (P<0.01). TNFα response to LPS was decreased in all patients (median 319 pg/mL versus controls 1256 pg/mL; P<0.01) and lowest in patients with sepsis or septic shock (median 128 pg/mL; P<0.01). HLA-DRA correlated positively with TNFα response in all study participants (r +0.60, P<0.001) and within patients (r +0.67, P<0.001). The TNFα:HLA-DRA ratio correlated negatively with severity and the Sequential Organ Failure Assessment (SOFA) score (Spearman's rho -0.59, P<0.001).
Conclusion: In this study, HLA-DRA expression was associated with a functional assay of the innate immune response. Future interventional studies aimed at the immune response during sepsis could make use of these methods for optimizing target groups based on biological plausibility and intervention effectiveness.
Conflict of interest statement
Figures



References
-
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–75. doi: 10.1016/j.immuni.2014.04.001 . - DOI - PubMed
-
- Hamers L, Kox M, Pickkers P. Sepsis-induced immunoparalysis: mechanisms, markers, and treatment options. Minerva anestesiologica. 2015;81(4):426–39. . - PubMed
-
- Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: Is individualized immuno-modulatory therapy the answer? Virulence. 2013;5(1). Epub 2013/09/27. doi: 10.4161/viru.26516 - DOI - PMC - PubMed
-
- Wiedermann CJ. Adjuvant treatment of sepsis: what is known? Medizinische Klinik, Intensivmedizin und Notfallmedizin. 2014;109(8):583–90. doi: 10.1007/s00063-014-0379-7 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

