IL-25-induced activation of nasal fibroblast and its association with the remodeling of chronic rhinosinusitis with nasal polyposis
- PMID: 28771607
- PMCID: PMC5542454
- DOI: 10.1371/journal.pone.0181806
IL-25-induced activation of nasal fibroblast and its association with the remodeling of chronic rhinosinusitis with nasal polyposis
Abstract
Background and objective: Interleukin (IL)-25 has been shown to play an important role in the pathogenesis of chronic rhinosinusitis with nasal polyps. Nasal polyps are associated with chronic inflammation of the mucous membranes in the paranasal sinuses and are involved in extracellular matrix (ECM) accumulation. The aim of this study is to evaluate the effects of IL-25 on myofibroblast differentiation, ECM production and the expression of matrix metalloproteinases in nasal polyp derived fibroblasts (NPDFs) and to determine the molecular mechanism underlying these processes.
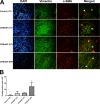
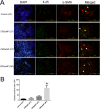
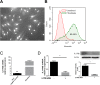
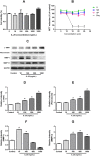
Materials and methods: A total of 40 patients were enrolled in this study for Immunofluorescence studies. Expression of IL17 receptor B was evaluated by real time reverse transcription polymerase chain reaction (PCR) in NPDFs. NPDFs were stimulated with IL-25 for 48 h in the presence or absence of mitogen-activated protein kinase (MAPK) and NF-κB inhibitors or small interfering RNAs (siRNA). The protein levels of fibrosis active mediators were examined using western blotting. Fibroblast migration was evaluated with a scratch assay. The total collagen amount was analyzed with the Sircol collagen assay.
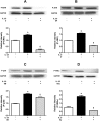
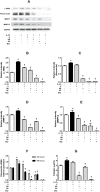
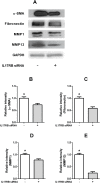
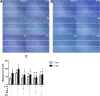
Results: IL-25 induced α-SMA, fibronectin, and MMP-1 and -13, which were dependent on IL-17RB. IL-25 also induced activation of NF-κB and mitogen-activated protein kinase (MAPKs). By using the specific inhibitor of ERK, p38, JNK and NF-κB (U, SB, SP and Bay), we found that IL-25-induced expressions of α-SMA, fibronectin, and MMPs was regulated by the signaling pathways of MAPKs and NF-κB. IL-25 also induces α-SMA, fibronectin, and MMPs expression through IL-17RB-dependent pathways in NPDFs. The increased migration ability induced by IL-25 was suppressed by the specific inhibitors of MAPKs and NF-κB.
Conclusion: Our data indicate that IL-25 induced myofibroblast differentiation, fibronectin production, and MMP-1 and -13 expressions through the signaling pathways of MAPKs and NF-κB. in NPDFs and increased expression of IL-25 were also involved in the pathogenesis of nasal polyposis by affecting nasal fibroblasts in chronic rhinosinusitis with nasal polyps.
Conflict of interest statement
Figures
References
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology Supplement. 2012;(23):3 p preceding table of contents, 1–298. Epub 2012/07/07. . - PubMed
-
- Redington AE. Fibrosis and airway remodelling. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2000;30 Suppl 1:42–5. Epub 2000/06/13. . - PubMed
-
- Xu J, Han R, Kim DW, Mo JH, Jin Y, Rha KS, et al. Role of Interleukin-10 on Nasal Polypogenesis in Patients with Chronic Rhinosinusitis with Nasal Polyps. PloS one. 2016;11(9):e0161013 Epub 2016/09/02. doi: 10.1371/journal.pone.0161013 ; PubMed Central PMCID: PMCPMC5008817. - DOI - PMC - PubMed
-
- Gabasa M, Royo D, Molina-Molina M, Roca-Ferrer J, Pujols L, Picado C, et al. Lung myofibroblasts are characterized by down-regulated cyclooxygenase-2 and its main metabolite, prostaglandin E2. PloS one. 2013;8(6):e65445 Epub 2013/06/12. doi: 10.1371/journal.pone.0065445 ; PubMed Central PMCID: PMCPMC3670886. - DOI - PMC - PubMed
-
- Watelet JB, Van Zele T, Gjomarkaj M, Canonica GW, Dahlen SE, Fokkens W, et al. Tissue remodelling in upper airways: where is the link with lower airway remodelling? Allergy. 2006;61(11):1249–58. Epub 2006/09/28. doi: 10.1111/j.1398-9995.2006.01226.x . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

