Deletion of Batf3-dependent antigen-presenting cells does not affect atherosclerotic lesion formation in mice
- PMID: 28771609
- PMCID: PMC5542449
- DOI: 10.1371/journal.pone.0181947
Deletion of Batf3-dependent antigen-presenting cells does not affect atherosclerotic lesion formation in mice
Abstract
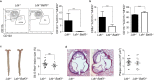
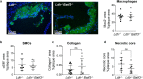
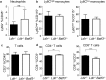
Atherosclerosis is the main underlying cause for cardiovascular events such as myocardial infarction and stroke and its development might be influenced by immune cells. Dendritic cells (DCs) bridge innate and adaptive immune responses by presenting antigens to T cells and releasing a variety of cytokines. Several subsets of DCs can be discriminated that engage specific transcriptional pathways for their development. Basic leucine zipper transcription factor ATF-like 3 (Batf3) is required for the development of classical CD8α+ and CD103+ DCs. By crossing mice deficient in Batf3 with atherosclerosis-prone low density lipoprotein receptor (Ldlr-/-)-deficient mice we here aimed to further address the contribution of Batf3-dependent CD8α+ and CD103+ antigen-presenting cells to atherosclerosis. We demonstrate that deficiency in Batf3 entailed mild effects on the immune response in the spleen but did not alter atherosclerotic lesion formation in the aorta or aortic root, nor affected plaque phenotype in low density lipoprotein receptor-deficient mice fed a high fat diet. We thus provide evidence that Batf3-dependent antigen-presenting cells do not have a prominent role in atherosclerosis.
Conflict of interest statement
Figures

Similar articles
-
Concise review: The heterogenous roles of BATF3 in cancer oncogenesis and dendritic cells and T cells differentiation and function considering the importance of BATF3-dependent dendritic cells.Immunogenetics. 2024 Apr;76(2):75-91. doi: 10.1007/s00251-024-01335-x. Epub 2024 Feb 15. Immunogenetics. 2024. PMID: 38358555 Review.
-
Ablation of CD8α(+) dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice.Sci Rep. 2015 Oct 21;5:15414. doi: 10.1038/srep15414. Sci Rep. 2015. PMID: 26486587 Free PMC article.
-
Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice.Eur J Immunol. 2015 Jan;45(1):119-29. doi: 10.1002/eji.201444651. Epub 2014 Nov 28. Eur J Immunol. 2015. PMID: 25312824 Free PMC article.
-
Batf3-independent langerin- CX3CR1- CD8α+ splenic DCs represent a precursor for classical cross-presenting CD8α+ DCs.J Leukoc Biol. 2014 Dec;96(6):1001-10. doi: 10.1189/jlb.1A0314-130R. Epub 2014 Aug 28. J Leukoc Biol. 2014. PMID: 25170118
-
Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
Cited by
-
Concise review: The heterogenous roles of BATF3 in cancer oncogenesis and dendritic cells and T cells differentiation and function considering the importance of BATF3-dependent dendritic cells.Immunogenetics. 2024 Apr;76(2):75-91. doi: 10.1007/s00251-024-01335-x. Epub 2024 Feb 15. Immunogenetics. 2024. PMID: 38358555 Review.
-
The Evolving Role of Dendritic Cells in Atherosclerosis.Int J Mol Sci. 2024 Feb 19;25(4):2450. doi: 10.3390/ijms25042450. Int J Mol Sci. 2024. PMID: 38397127 Free PMC article. Review.
-
cDC1s Promote Atherosclerosis via Local Immunity and Are Targetable for Therapy.Circ Res. 2025 Jul 18;137(3):400-416. doi: 10.1161/CIRCRESAHA.124.325792. Epub 2025 May 30. Circ Res. 2025. PMID: 40444360 Free PMC article.
-
Local adaptive immunity in atherosclerosis with T cell activation by aortic dendritic cells accelerates pathogenesis.iScience. 2024 Oct 10;27(11):111144. doi: 10.1016/j.isci.2024.111144. eCollection 2024 Nov 15. iScience. 2024. PMID: 39502289 Free PMC article.
-
Immunobiology of Atherosclerosis: A Complex Net of Interactions.Int J Mol Sci. 2019 Oct 24;20(21):5293. doi: 10.3390/ijms20215293. Int J Mol Sci. 2019. PMID: 31653058 Free PMC article. Review.
References
-
- Gimbrone MA Jr., Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 2016,118:620–636. doi: 10.1161/CIRCRESAHA.115.306301 - DOI - PMC - PubMed
-
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006,6:508–519. doi: 10.1038/nri1882 - DOI - PubMed
-
- Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2000,20:10–17. - PubMed
-
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986,6:131–138. - PubMed
-
- Zernecke A. Dendritic cells in atherosclerosis: evidence in mice and humans. Arterioscler Thromb Vasc Biol 2015,35:763–770. doi: 10.1161/ATVBAHA.114.303566 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

