Quantification of transplant-derived circulating cell-free DNA in absence of a donor genotype
- PMID: 28771616
- PMCID: PMC5542400
- DOI: 10.1371/journal.pcbi.1005629
Quantification of transplant-derived circulating cell-free DNA in absence of a donor genotype
Abstract
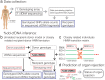
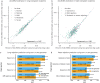
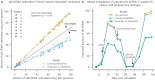
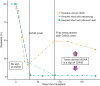
Quantification of cell-free DNA (cfDNA) in circulating blood derived from a transplanted organ is a powerful approach to monitoring post-transplant injury. Genome transplant dynamics (GTD) quantifies donor-derived cfDNA (dd-cfDNA) by taking advantage of single-nucleotide polymorphisms (SNPs) distributed across the genome to discriminate donor and recipient DNA molecules. In its current implementation, GTD requires genotyping of both the transplant recipient and donor. However, in practice, donor genotype information is often unavailable. Here, we address this issue by developing an algorithm that estimates dd-cfDNA levels in the absence of a donor genotype. Our algorithm predicts heart and lung allograft rejection with an accuracy that is similar to conventional GTD. We furthermore refined the algorithm to handle closely related recipients and donors, a scenario that is common in bone marrow and kidney transplantation. We show that it is possible to estimate dd-cfDNA in bone marrow transplant patients that are unrelated or that are siblings of the donors, using a hidden Markov model (HMM) of identity-by-descent (IBD) states along the genome. Last, we demonstrate that comparing dd-cfDNA to the proportion of donor DNA in white blood cells can differentiate between relapse and the onset of graft-versus-host disease (GVHD). These methods alleviate some of the barriers to the implementation of GTD, which will further widen its clinical application.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Benden C, Edwards LB, Kucheryavaya AY, Christie JD, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report—2013; focus theme: age. J Heart Lung Transplant. 2013;32: 989–97. doi: 10.1016/j.healun.2013.08.008 - DOI - PubMed
-
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report—2011. J Heart Lung Transplant. 2011;30: 1078–94. doi: 10.1016/j.healun.2011.08.003 - DOI - PubMed
-
- Bloom RD, Goldberg LR, Wang AY, Faust TW, Kotloff RM. An overview of solid organ transplantation. Clin Chest Med. 2005;26: 529–43, v doi: 10.1016/j.ccm.2005.06.002 - DOI - PubMed
-
- Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11: 1226–35. doi: 10.1111/j.1600-6143.2011.03539.x - DOI - PubMed
-
- Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report—2013; focus theme: age. J Heart Lung Transplant. 2013;32: 965–78. doi: 10.1016/j.healun.2013.08.007 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

