Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: A placebo-controlled randomized trial
- PMID: 28771627
- PMCID: PMC5542589
- DOI: 10.1371/journal.pone.0182411
Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: A placebo-controlled randomized trial
Abstract
Background: Antibiotics are frequently used to treat wheezing children. Macrolides may be effective in treating bronchiolitis and asthma.
Method: We completed a prospective, double-blinded, randomized placebo-control trial of azithromycin among pre-school children (12 to 60 months of age) presenting to the emergency department with wheeze. Patients were randomized to receive either five days of azithromycin or placebo. Primary outcome was time to resolution of respiratory symptoms after treatment initiation. Secondary outcomes included the number of days children used a Short-Acting Beta-Agonists during the 21 day follow-up and time to disease exacerbation during the following six months (unscheduled health care visit or treatment with an oral corticosteroid for acute respiratory symptoms).
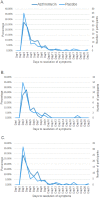
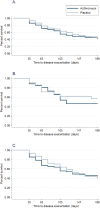
Results: Of the 300 wheezing children recruited, 222 and 169 were analyzed for the primary and secondary outcomes, respectively. The treatment groups had similar demographics and clinical parameters at baseline. Median time to resolution of respiratory symptoms was four days for both treatment arms (interquartile range (IQR) 3,6; p = 0.28). Median number of days of Short-Acting Beta-Agonist use among those who received azithromycin was four and a half days (IQR 2, 7) and five days (IQR 2, 9; p = 0.22) among those who received placebo. Participants who received azithromycin had a 0.91 hazard ratio for time to six-month exacerbation compared to placebo (95% CI 0.61, 1.36, p = 0.65). A pre-determined subgroup analysis showed no differences in outcomes for children with their first or repeat episode of wheezing. There was no significant difference in the proportion of participants experiencing an adverse event.
Conclusion: Azithromycin neither reduced duration of respiratory symptoms nor time to respiratory exacerbation in the following six months after treatment among wheezing preschool children presenting to an emergency department. There was no significant effect among children with either first-time or prior wheezing.
Conflict of interest statement
Figures

References
-
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and Wheezing in the First Six Years of Life. N Engl J Med. 1995;332: 133–138. doi: 10.1056/NEJM199501193320301 - DOI - PubMed
-
- Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007;42: 723–728. doi: 10.1002/ppul.20644 - DOI - PubMed
-
- Frank Mo, Robinson C, Choi BC, Li FC. Childhood asthma management and control. Analysis of the Student Lung Health Survey (SLHS) database, Canada 1996. Int J Adolesc Med Health. 2004;16 doi: 10.1515/ijamh.2004.16.1.29 - DOI - PubMed
-
- Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. The Lancet. 2014;383: 1593–1604. doi: 10.1016/s0140-6736(14)60615-2 - DOI - PubMed
-
- Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. PEDIATRICS. 2014;134: e1474–e1502. doi: 10.1542/peds.2014-2742 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

