Characterization of primary rat nasal epithelial cultures in CFTR knockout rats as a model for CF sinus disease
- PMID: 28771736
- PMCID: PMC5654659
- DOI: 10.1002/lary.26720
Characterization of primary rat nasal epithelial cultures in CFTR knockout rats as a model for CF sinus disease
Abstract
Objective: The objectives of the current experiments were to develop and characterize primary rat nasal epithelial cultures and evaluate their usefulness as a model of cystic fibrosis (CF) sinonasal transepithelial transport and CF transmembrane conductance regulator (CFTR) function.
Study design: Laboratory in vitro and animal studies.
Methods: CFTR+/+ and CFTR-/- rat nasal septal epithelia (RNSE) were cultured on semipermeable supports at an air-liquid interface to confluence and full differentiation. Monolayers were mounted in Ussing chambers for pharmacologic manipulation of ion transport and compared to similar filters containing murine (MNSE) and human (HSNE) epithelia. Histology and scanning electron microscopy (SEM) were completed. Real-time polymerase chain reaction of CFTR+/+ RNSE, MNSE, and HSNE was performed to evaluate relative CFTR gene expression.
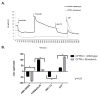
Results: Forskolin-stimulated anion transport (ΔIsc in μA/cm2 ) was significantly greater in epithelia derived from CFTR+/+ when compared to CFTR-/- animals (100.9 ± 3.7 vs. 10.5 ± 0.9; P < 0.0001). Amiloride-sensitive ISC was equivalent (-42.3 ± 2.8 vs. -46.1 ± 2.3; P = 0.524). No inhibition of CFTR-mediated chloride (Cl- ) secretion was exhibited in CFTR-/- epithelia with the addition of the specific CFTR inhibitor, CFTRInh -172. However, calcium-activated Cl- secretion (UTP) was significantly increased in CFTR-/- RNSE (CFTR-/- -106.8 ± 1.6 vs. CFTR+/+ -32.2 ± 3.1; P < 0.0001). All responses were larger in RNSE when compared to CFTR+/+ and CFTR-/- (or F508del/F508del) murine and human cells (P < 0.0001). Scanning electron microscopy demonstrated 80% to 90% ciliation in all RNSE cultures. There was no evidence of infection in CFTR-/- rats at 4 months. CFTR expression was similar among species.
Conclusion: The successful development of the CFTR-/- rat enables improved evaluation of CF sinus disease based on characteristic abnormalities of ion transport.
Level of evidence: NA. Laryngoscope, 127:E384-E391, 2017.
Keywords: CFTR; Cystic fibrosis; Ussing chamber; chronic rhinosinusitis; chronic sinusitis; electrophysiology; ion transport; mucociliary clearance; rat nasal culture; sinusitis.
© 2017 The American Laryngological, Rhinological and Otological Society, Inc.
Figures





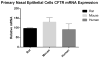
Similar articles
-
Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency.Laryngoscope. 2015 Oct;125 Suppl 7(0 7):S1-S13. doi: 10.1002/lary.25335. Epub 2015 May 6. Laryngoscope. 2015. PMID: 25946147 Free PMC article.
-
Porcine nasal epithelial cultures for studies of cystic fibrosis sinusitis.Int Forum Allergy Rhinol. 2014 Jul;4(7):565-70. doi: 10.1002/alr.21335. Epub 2014 Apr 14. Int Forum Allergy Rhinol. 2014. PMID: 24733748 Free PMC article.
-
Chlorogenic Acid Activates CFTR-Mediated Cl- Secretion in Mice and Humans: Therapeutic Implications for Chronic Rhinosinusitis.Otolaryngol Head Neck Surg. 2015 Aug;153(2):291-7. doi: 10.1177/0194599815586720. Epub 2015 May 27. Otolaryngol Head Neck Surg. 2015. PMID: 26019132 Free PMC article.
-
Transepithelial nasal potential difference (NPD) measurements in cystic fibrosis (CF).Med Wieku Rozwoj. 2013 Jan-Mar;17(1):13-7. Med Wieku Rozwoj. 2013. PMID: 23749691 Review.
-
Cystic Fibrosis Sinusitis.Adv Otorhinolaryngol. 2016;79:29-37. doi: 10.1159/000444959. Epub 2016 Jul 28. Adv Otorhinolaryngol. 2016. PMID: 27466844 Review.
Cited by
-
Animal Models in the Pathophysiology of Cystic Fibrosis.Front Pharmacol. 2019 Jan 4;9:1475. doi: 10.3389/fphar.2018.01475. eCollection 2018. Front Pharmacol. 2019. PMID: 30662403 Free PMC article. Review.
-
Assessing the consistency of iPSC and animal models in cystic fibrosis modelling: A meta-analysis.PLoS One. 2022 Aug 9;17(8):e0272091. doi: 10.1371/journal.pone.0272091. eCollection 2022. PLoS One. 2022. PMID: 35944004 Free PMC article.
-
Red ginseng aqueous extract improves mucociliary transport dysfunction and histopathology in CF rat airways.J Cyst Fibros. 2023 Nov;22(6):1113-1119. doi: 10.1016/j.jcf.2023.09.002. Epub 2023 Sep 12. J Cyst Fibros. 2023. PMID: 37704464 Free PMC article.
-
Seeing cilia: imaging modalities for ciliary motion and clinical connections.Am J Physiol Lung Cell Mol Physiol. 2018 Jun 1;314(6):L909-L921. doi: 10.1152/ajplung.00556.2017. Epub 2018 Mar 1. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29493257 Free PMC article. Review.
-
Characterization of two rat models of cystic fibrosis-KO and F508del CFTR-Generated by Crispr-Cas9.Animal Model Exp Med. 2019 Nov 25;2(4):297-311. doi: 10.1002/ame2.12091. eCollection 2019 Dec. Animal Model Exp Med. 2019. PMID: 31942562 Free PMC article.
References
-
- Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep. 2004;53:1–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

