Phase I/II study of pralatrexate in Japanese patients with relapsed or refractory peripheral T-cell lymphoma
- PMID: 28771889
- PMCID: PMC5623731
- DOI: 10.1111/cas.13340
Phase I/II study of pralatrexate in Japanese patients with relapsed or refractory peripheral T-cell lymphoma
Abstract
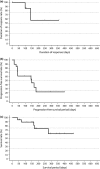
Pralatrexate is a novel antifolate approved in the USA for the treatment of relapsed or refractory peripheral T-cell lymphoma. To assess its safety, efficacy, and pharmacokinetics in Japanese patients with this disease, we undertook a phase I/II study. Pralatrexate was given i.v. weekly for 6 weeks of a 7-week cycle. All patients received concurrent vitamin B12 and folic acid. In phase I, three patients received pralatrexate 30 mg/m2 and none experienced a dose-limiting toxicity. In phase II, we treated 22 additional patients with that dose. The median number of treatment cycles was 1 (range, 1-9). Nine of 20 evaluable patients (45%) achieved an objective response by central review, including two complete responses. All responses occurred within the first treatment cycle. At the time of data cut-off, median progression-free survival was 150 days. Median overall survival was not reached. In the total population, the most commonly reported adverse events included mucositis (88%), thrombocytopenia (68%), liver function test abnormality (64%), anemia (60%), and lymphopenia (56%). Grade 3/4 adverse events included lymphopenia (52%), thrombocytopenia (40%), leukopenia (28%), neutropenia (24%), anemia (20%), and mucositis (20%). The pharmacokinetic profile showed no drug accumulation with repeat dosing. These results indicate that pralatrexate is generally well tolerated and effective in Japanese patients with relapsed or refractory peripheral T-cell lymphoma. This trial was registered with ClinicalTrials.gov (NCT02013362).
Keywords: Clinical trial; Japanese; folic acid antagonists; peripheral T-cell lymphoma; pralatrexate.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Vose J, Armitage J, Weisenburger D; International T‐Cell Lymphoma Project . International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–30. - PubMed
-
- Park S, Ko YH. Peripheral T cell lymphoma in Asia. Int J Hematol 2014; 99: 227–39. - PubMed
-
- Kitahara H, Maruyama D, Maeshima AM et al Prognosis of patients with peripheral T cell lymphoma who achieve complete response after CHOP/CHOP‐like chemotherapy without autologous stem cell transplantation as an initial treatment. Ann Hematol 2017; 96: 411–20. - PubMed
-
- Mak V, Hamm J, Chhanabhai M et al Survival of patients with peripheral T‐cell lymphoma after first relapse or progression: spectrum of disease and rare long‐term survivors. J Clin Oncol 2013; 31: 1970–6. - PubMed
-
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN Guidelines®): T‐cell lymphomas, version 2.2017 – February 21, 2017. [Cited 10 Mar 2017.] Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

