Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study
- PMID: 28771923
- PMCID: PMC5811811
- DOI: 10.1111/dom.13077
Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study
Abstract
Aims: To compare the sodium-glucose-cotransporter-2 (SGLT-2) inhibitor dapagliflozin with dipeptidyl peptidase-4 (DPP-4) inhibitors with regard to risk associations with major adverse cardiovascular (CV) events (MACE; non-fatal myocardial infarction, non-fatal stroke or cardiovascular mortality), hospitalization for heart failure (HHF), atrial fibrillation and severe hypoglycaemia in patients with type 2 diabetes (T2D) in a real-world setting.
Methods: All patients with T2D prescribed glucose-lowering drugs (GLDs) during 2012 to 2015 were identified in nationwide registries in Denmark, Norway and Sweden. Patients were divided into two groups: new users of dapagliflozin and new users of DPP-4 inhibitors, matched 1:3 by propensity score, calculated by patient characteristics, comorbidities and drug treatment. Cox survival models were used to estimate hazard ratio (HR) per country separately, and a weighted average was calculated.
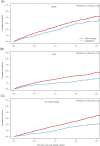
Results: After matching, a total of 40 908 patients with T2D were identified as new users of dapagliflozin (n = 10 227) or a DPP-4 inhibitor (n = 30 681). The groups were well balanced at baseline; their mean age was 61 years and 23% had CV disease. The mean follow-up time was 0.95 years, with a total of 38 760 patient-years. Dapagliflozin was associated with a lower risk of MACE, HHF and all-cause mortality compared with DPP-4 inhibitors: HRs 0.79 (95% confidence interval [CI] 0.67-0.94), 0.62 (95% CI 0.50-0.77), and 0.59 (95% CI 0.49-0.72), respectively. Numerically lower, but non-significant HRs were observed for myocardial infarction (0.91 [95% CI 0.72-1.16]), stroke (0.79 [95% CI 0.61-1.03]) and CV mortality (0.76 [95% CI 0.53-1.08]) Neutral associations with atrial fibrillation and severe hypoglycaemia were observed.
Conclusions: Dapagliflozin was associated with lower risks of CV events and all-cause mortality compared with DPP-4 inhibitors in a real-world clinical setting and a broad T2D population.
Keywords: DPP-4 inhibitor; cardiovascular disease; dapagliflozin; diabetes complications; hypoglycaemia; type 2 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
F. P. reports having received research grants from AstraZeneca and Novartis and lecture fees from Novartis, Eli Lilly, MSD, AstraZeneca and Boehringer Ingelheim and having served as a consultant for Astra Zeneca, Amgen, Novo Nordisk and MSD. T. N. has received unrestricted grants from AstraZeneca and Novo Nordisk, and is on the national board of Novo Nordisk, Sanofi‐Aventis, Eli Lilly and Boehringer Ingelheim. M. E. J. holds shares in Novo Nordisk and has received grants and lecture fees from Astra Zeneca. B. C. is a shareholder of Novo Nordisk. J. W. E. has received honoraria or research grants from AstraZeneca, Novo Nordisk, Bristol‐Myers‐Squibb, Sanofi and MSD. P. F. holds a full‐time position at AstraZeneca. D. N. has received consultancy fees from Novo Nordisk, Astra Zeneca and Eli Lilly. M. T. is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, for which AstraZeneca Nordic‐Baltic is a client. H. L. G. reports honoraria from Sanofi, Novo Nordisk, Lilly, Boehringer Ingelheim. A. N. has honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim, Novo Nordisk. J. B. holds a full‐time position at AstraZeneca as epidemiologist. K. I. B. reports grants to his institution from AstraZeneca for this study and for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme.
Figures
References
-
- Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose‐lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006‐2013. Diabetologia. 2016;59(8):1692–1701. - PubMed
-
- Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1611925. [Epub ahead of print]. - DOI - PubMed
-
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. - PubMed
-
- The CVD‐REAL Investigators and Study Group. CVD‐REAL: a multinational, retrospective, observational study in patients with type 2 diabetes mellitus who are initiating treatment with SGLT‐2 inhibitor or another glucose‐lowering drug. AstraZeneca; 2017. https://www.cvdreal.com/. Accessed March 10, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

