Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides
- PMID: 28772020
- PMCID: PMC5658588
- DOI: 10.1111/1751-7915.12799
Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides
Abstract
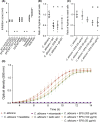
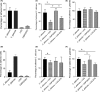
A number of clinical studies have shown protective effects of lactobacilli against Candida species in the gastrointestinal tract, the urogenital tract and the oral cavity, while others did not show clear effects. Evidence on the mode of action of lactobacilli against Candida is also still lacking. In this study, the anti-Candida activity of the model probiotic strain Lactobacillus rhamnosus GG was explored in different assays to determine molecular interactions. We found that L. rhamnosus GG was able to interfere with Candida growth, morphogenesis and adhesion. These three aspects of Candida's physiology are all crucial to its opportunistic pathogenesis. In follow-up assays, we compared the activity of L. rhamnosus GG wild-type with its exopolysaccharide (EPS)-deficient mutant and purified EPS to evaluate the involvement of this outer carbohydrate layer. Our data demonstrate that purified EPS can both interfere with hyphal formation and adhesion to epithelial cells, which indicates that EPS is part of a combined molecular mechanism underlying the antihyphal and anti-adhesion mechanisms of L. rhamnosus GG.
© 2017 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures
Similar articles
-
An Exopolysaccharide-Deficient Mutant of Lactobacillus rhamnosus GG Efficiently Displays a Protective Llama Antibody Fragment against Rotavirus on Its Surface.Appl Environ Microbiol. 2015 Sep 1;81(17):5784-93. doi: 10.1128/AEM.00945-15. Epub 2015 Jun 19. Appl Environ Microbiol. 2015. PMID: 26092449 Free PMC article.
-
Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG.Sci Rep. 2019 Feb 27;9(1):2900. doi: 10.1038/s41598-019-39625-0. Sci Rep. 2019. PMID: 30814593 Free PMC article.
-
In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates.J Appl Microbiol. 2015 Nov;119(5):1383-90. doi: 10.1111/jam.12947. Epub 2015 Oct 5. J Appl Microbiol. 2015. PMID: 26335148
-
Adaptation factors of the probiotic Lactobacillus rhamnosus GG.Benef Microbes. 2010 Nov;1(4):335-42. doi: 10.3920/BM2010.0032. Benef Microbes. 2010. PMID: 21831772 Review.
-
Lactobacillus rhamnosus sepsis associated with probiotic therapy in an extremely preterm infant: Pathogenesis and a review for clinicians.J Microbiol Immunol Infect. 2021 Aug;54(4):575-580. doi: 10.1016/j.jmii.2020.03.029. Epub 2020 Apr 3. J Microbiol Immunol Infect. 2021. PMID: 32307246 Review.
Cited by
-
Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi.Biomimetics (Basel). 2024 Jul 12;9(7):425. doi: 10.3390/biomimetics9070425. Biomimetics (Basel). 2024. PMID: 39056866 Free PMC article. Review.
-
Exopolysaccharides from vaginal lactobacilli modulate microbial biofilms.Microb Cell Fact. 2023 Mar 8;22(1):45. doi: 10.1186/s12934-023-02053-x. Microb Cell Fact. 2023. PMID: 36890519 Free PMC article.
-
Anticandidal and Antibiofilm Effect of Synbiotics including Probiotics and Inulin-Type Fructans.Antibiotics (Basel). 2022 Aug 21;11(8):1135. doi: 10.3390/antibiotics11081135. Antibiotics (Basel). 2022. PMID: 36010004 Free PMC article.
-
Reuterin, Phenyllactic Acid, and Exopolysaccharides as Main Antifungal Molecules Produced by Lactic Acid Bacteria: A Scoping Review.Foods. 2024 Feb 29;13(5):752. doi: 10.3390/foods13050752. Foods. 2024. PMID: 38472865 Free PMC article.
-
Interactions between Candida albicans and the resident microbiota.Front Microbiol. 2022 Sep 20;13:930495. doi: 10.3389/fmicb.2022.930495. eCollection 2022. Front Microbiol. 2022. PMID: 36204612 Free PMC article. Review.
References
-
- Calderone, R. A. , and Fonzi, W. A. (2001) Virulence factors of Candida albicans. Trends Microbiol 9: 327–335. - PubMed
-
- De Keersmaecker, S. C. J. , Verhoeven, T. L. , Desair, J. , Marchal, K. , Vanderleyden, J. , and Nagy, I. (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 259: 89–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

