Collectin-11 Is an Important Modulator of Retinal Pigment Epithelial Cell Phagocytosis and Cytokine Production
- PMID: 28772263
- PMCID: PMC6738776
- DOI: 10.1159/000478042
Collectin-11 Is an Important Modulator of Retinal Pigment Epithelial Cell Phagocytosis and Cytokine Production
Abstract
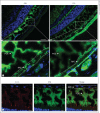
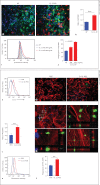
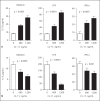
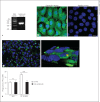
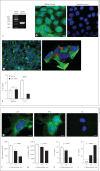
In this paper, we report previously unknown roles for collectin-11 (CL-11, a soluble C-type lectin) in modulating the retinal pigment epithelial (RPE) cell functions of phagocytosis and cytokine production. We found that CL-11 and its carbohydrate ligand are expressed in both the murine and human neural retina; these resemble each other in terms of RPE and photoreceptor cells. Functional analysis of murine RPE cells showed that CL-11 facilitates the opsonophagocytosis of photoreceptor outer segments and apoptotic cells, and also upregulates IL-10 production. Mechanistic analysis revealed that calreticulin on the RPE cells is required for CL-11-mediated opsonophagocytosis whereas signal-regulatory protein α and mannosyl residues on the cells are involved in the CL-11-mediated upregulation of IL-10 production. This study is the first to demonstrate the role of CL-11 and the molecular mechanisms involved in modulating RPE cell phagocytosis and cytokine production. It provides a new insight into retinal health and disease and has implications for other phagocytic cells.
Keywords: CL-K1; Collectin 11; Cytokine production; Opsonophagocytosis; Retinal pigment epithelial cells.
© 2017 S. Karger AG, Basel.
Figures

References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. - PubMed
-
- Keshi H, Sakamoto T, Kawai T, Ohtani K, Katoh T, Jang SJ, Motomura W, Yoshizaki T, Fukuda M, Koyama S, Fukuzawa J, Fukuoh A, Yoshida I, Suzuki Y, Wakamiya N. Identification and characterization of a novel human collectin CL-K1. Microbiol Immunol. 2006;50:1001–1013. - PubMed
-
- Ohtani K, Suzuki Y, Eda S, Kawai T, Kase T, Yamazaki H, Shimada T, Keshi H, Sakai Y, Fukuoh A, Sakamoto T, Wakamiya N. Molecular cloning of a novel human collectin from liver (CL-L1) J Biol Chem. 1999;274:13681–13689. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

