Biogenic and Synthetic Peptides with Oppositely Charged Amino Acids as Binding Sites for Mineralization
- PMID: 28772478
- PMCID: PMC5459154
- DOI: 10.3390/ma10020119
Biogenic and Synthetic Peptides with Oppositely Charged Amino Acids as Binding Sites for Mineralization
Abstract
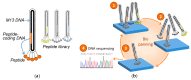
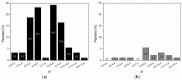
Proteins regulate diverse biological processes by the specific interaction with, e.g., nucleic acids, proteins and inorganic molecules. The generation of inorganic hybrid materials, such as shell formation in mollusks, is a protein-controlled mineralization process. Moreover, inorganic-binding peptides are attractive for the bioinspired mineralization of non-natural inorganic functional materials for technical applications. However, it is still challenging to identify mineral-binding peptide motifs from biological systems as well as for technical systems. Here, three complementary approaches were combined to analyze protein motifs consisting of alternating positively and negatively charged amino acids: (i) the screening of natural biomineralization proteins; (ii) the selection of inorganic-binding peptides derived from phage display; and (iii) the mineralization of tobacco mosaic virus (TMV)-based templates. A respective peptide motif displayed on the TMV surface had a major impact on the SiO₂ mineralization. In addition, similar motifs were found in zinc oxide- and zirconia-binding peptides indicating a general binding feature. The comparative analysis presented here raises new questions regarding whether or not there is a common design principle based on acidic and basic amino acids for peptides interacting with minerals.
Keywords: Inorganic-binding peptides; biomineralization; phage display; silicification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brena B.M., Batista-Viera F. Immobilization of Enzymes. In: Guisán J.M., editor. Immobilization of Enzymes and Cells. Volume 22. Humana Press; New York, NY, USA: 2006. pp. 15–30.
-
- Rothenstein D., Facey S.J., Ploss M., Hans P., Melcher M., Srot V., van Aken P.A., Hauer B., Bill J. Mineralization of gold nanoparticles using tailored M13 phages. Bioinsp. Biomim. Nanobiomater. 2013;2:173–185. doi: 10.1680/bbn.13.00004. - DOI
-
- Rothenstein D., Shopova-Gospodinova D., Bakradze G., Jeurgens L.P.H., Bill J. Generation of luminescence in biomineralized zirconia by zirconia-binding peptides. CrystEngComm. 2015;17:1783–1790. doi: 10.1039/C4CE01510J. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

