Cytotoxicity of Light-Cured Dental Materials according to Different Sample Preparation Methods
- PMID: 28772647
- PMCID: PMC5503327
- DOI: 10.3390/ma10030288
Cytotoxicity of Light-Cured Dental Materials according to Different Sample Preparation Methods
Abstract
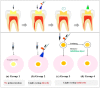
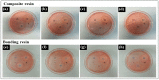
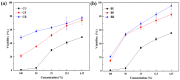
Dental light-cured resins can undergo different degrees of polymerization when applied in vivo. When polymerization is incomplete, toxic monomers may be released into the oral cavity. The present study assessed the cytotoxicity of different materials, using sample preparation methods that mirror clinical conditions. Composite and bonding resins were used and divided into four groups according to sample preparation method: uncured; directly cured samples, which were cured after being placed on solidified agar; post-cured samples were polymerized before being placed on agar; and "removed unreacted layer" samples had their oxygen-inhibition layer removed after polymerization. Cytotoxicity was evaluated using an agar diffusion test, MTT assay, and confocal microscopy. Uncured samples were the most cytotoxic, while removed unreacted layer samples were the least cytotoxic (p < 0.05). In the MTT assay, cell viability increased significantly in every group as the concentration of the extracts decreased (p < 0.05). Extracts from post-cured and removed unreacted layer samples of bonding resin were less toxic than post-cured and removed unreacted layer samples of composite resin. Removal of the oxygen-inhibition layer resulted in the lowest cytotoxicity. Clinicians should remove unreacted monomers on the resin surface immediately after restoring teeth with light-curing resin to improve the restoration biocompatibility.
Keywords: biocompatibility; cytotoxicity; oxygen-inhibition layer; resin-based materials; sample preparation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

