Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications
- PMID: 28772697
- PMCID: PMC5506916
- DOI: 10.3390/ma10040334
Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications
Abstract
Calcium phosphate (CaP) bioceramics are widely used in the field of bone regeneration, both in orthopedics and in dentistry, due to their good biocompatibility, osseointegration and osteoconduction. The aim of this article is to review the history, structure, properties and clinical applications of these materials, whether they are in the form of bone cements, paste, scaffolds, or coatings. Major analytical techniques for characterization of CaPs, in vitro and in vivo tests, and the requirements of the US Food and Drug Administration (FDA) and international standards from CaP coatings on orthopedic and dental endosseous implants, are also summarized, along with the possible effect of sterilization on these materials. CaP coating technologies are summarized, with a focus on electrochemical processes. Theories on the formation of transient precursor phases in biomineralization, the dissolution and reprecipitation as bone of CaPs are discussed. A wide variety of CaPs are presented, from the individual phases to nano-CaP, biphasic and triphasic CaP formulations, composite CaP coatings and cements, functionally graded materials (FGMs), and antibacterial CaPs. We conclude by foreseeing the future of CaPs.
Keywords: bioceramics; biomineralization; bone cement; calcium phosphate; coating; composites; drug delivery; electrochemical deposition; functionally graded materials; nano-hydroxyapatite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
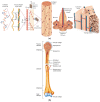
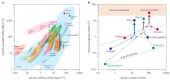
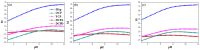

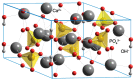








References
-
- Driskell T.D. Early history of calcium phosphate materials and coatings. In: Horowitz E., Parr J.E., editors. Characterization and Performance of Calcium Phosphate Coatings for Implants. American Society for Testing and Materials (ASTM); Philadelphia, PA, USA: 1994. pp. 1–9.
-
- Shackelford J.F. Bioceramics—An historical perspective. Mater. Sci. Forum. 1999;293:1–4. doi: 10.4028/www.scientific.net/MSF.293.1. - DOI
-
- Shepperd J. The early biological history of calcium phosphates. In: Epinette J.A., Manley M.T., editors. Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty. Springer; Paris, France: 2004. pp. 3–8.
-
- Leeuwenhoek A. Microscopical observations concerning blood, milk, bone, the brain, spittle, and cuticula, etc. Philos. Trans. 1674;9:121–128. doi: 10.1098/rstl.1674.0030. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

