A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †
- PMID: 28772698
- PMCID: PMC5506898
- DOI: 10.3390/ma10040338
A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †
Abstract
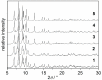
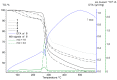
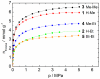
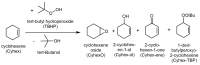
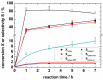
The synthesis and characterization of an isomorphous series of copper-containing microporous metal-organic frameworks (MOFs) based on triazolyl isophthalate linkers with the general formula [Cu₄(μ₃-OH)₂(R¹-R²-trz-ia)₃(H₂O)x] are presented. Through size adjustment of the alkyl substituents R¹ and/or R² at the linker, the impact of linker functionalization on structure-property relationships was studied. Due to the arrangement of the substituents towards the cavities, the porosity (pore fraction 28%-39%), as well as the pore size can be adjusted by the size of the substituents of the triazole ring. Thermal analysis and temperature-dependent PXRD studies reveal a thermal stability of the MOFs up to 230 °C due to increasing framework stability through fine-tuning of the linker substitution pattern. Adsorption of CO₂ (298 K) shows a decreasing maximum loading with increasing steric demand of the substituents of the triazole ring. Furthermore, the selective oxidation of cyclohexene with tert-butyl hydroperoxide (TBHP) is studied over the MOFs at 323 K in liquid chloroform. The catalytic activity increases with the steric demand of the substituents. Additionally, these isomorphous MOFs exhibit considerable robustness under oxidizing conditions confirmed by CO₂ adsorption studies, as well as by the catalytic selective oxidation experiments.
Keywords: crystal structures; cyclohexene oxidation; heterogeneous catalysis; linker substitution pattern; structure-property relationship; triazolyl isophthalate MOFs.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study. They further had no role in the collection, analyses or interpretation of data, nor in the writing of the manuscript, nor in the decision to publish the results.
Figures


References
-
- Lässig D., Lincke J., Moellmer J., Reichenbach C., Moeller A., Gläser R., Kalies G., Cychosz K.A., Thommes M., Staudt R., et al. A Microporous Copper Metal-Organic Framework with High H2 and CO2 Adsorption Capacity at Ambient Pressure. Angew. Chem. Int. Ed. 2011;50:10344–10348. doi: 10.1002/anie.201102329. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

