The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes
- PMID: 28772718
- PMCID: PMC5506934
- DOI: 10.3390/ma10040353
The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes
Abstract
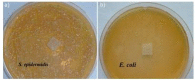
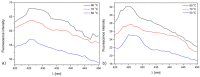
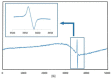
Surgical meshes were modified with zinc oxide (ZnO) using a chemical bath deposition method (CBD) at 50 °C, 70 °C, or 90 °C, in order to biologically activate them. Scanning electron microscopy (SEM), mass changes, and X-ray diffraction measurements revealed that at low temperatures Zn(OH)₂ was formed, and that this was converted into ZnO with a temperature increase. The antimicrobial activity without light stimulation of the ZnO modified Mersilene™ meshes was related to the species of microorganism, the incubation time, and the conditions of the experiment. Generally, cocci (S. aureus, S. epidermidis) and yeast (C. albicans) were more sensitive than Gram-negative rods (E. coli). The differences in sensitivity of the studied microorganisms to ZnO were discussed. The most active sample was that obtained at 90 °C. The mechanism of antimicrobial action of ZnO was determined by various techniques, such as zeta potential analysis, electron paramagnetic resonance (EPR) spectroscopy, SEM studies, and measurements of Zn(II) and reactive oxygen species (ROS) concentration. Our results confirmed that the generation of free radicals was crucial, which occurs on the surface of crystalline ZnO.
Keywords: antimicrobial properties; crystallization; free radicals; surgical mesh; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, and in the decision to publish the results.
Figures








Similar articles
-
Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties.Materials (Basel). 2018 Apr 30;11(5):707. doi: 10.3390/ma11050707. Materials (Basel). 2018. PMID: 29710873 Free PMC article.
-
Effect of bath temperature on the properties of nanocrystalline ZnO thin films.J Nanosci Nanotechnol. 2010 May;10(5):3412-5. doi: 10.1166/jnn.2010.2306. J Nanosci Nanotechnol. 2010. PMID: 20358968
-
Formation and distribution of ZnO nanoparticles and its effect on E. coli in the presence of sepiolite and silica within the chitosan matrix via sonochemistry.Ultrason Sonochem. 2017 Sep;38:720-725. doi: 10.1016/j.ultsonch.2016.08.027. Epub 2016 Aug 25. Ultrason Sonochem. 2017. PMID: 27614583
-
Insights into the antimicrobial mechanism of Ag and I incorporated ZnO nanoparticle derivatives under visible light.Mater Sci Eng C Mater Biol Appl. 2020 Feb;107:110220. doi: 10.1016/j.msec.2019.110220. Epub 2019 Oct 30. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31761246
-
Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism.Adv Colloid Interface Sci. 2017 Nov;249:37-52. doi: 10.1016/j.cis.2017.07.033. Epub 2017 Aug 26. Adv Colloid Interface Sci. 2017. PMID: 28923702 Review.
Cited by
-
Characterization and in vivo evaluation of a fabricated absorbable poly(vinyl alcohol)-based hernia mesh.Heliyon. 2023 Nov 15;9(11):e22279. doi: 10.1016/j.heliyon.2023.e22279. eCollection 2023 Nov. Heliyon. 2023. PMID: 38045132 Free PMC article.
-
Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles.Polymers (Basel). 2019 Mar 25;11(3):562. doi: 10.3390/polym11030562. Polymers (Basel). 2019. PMID: 30960546 Free PMC article.
-
Enhancement of Thermal Diffusivity in Phase-Separated Bismaleimide/Poly(ether imide) Composite Films Containing Needle-Shaped ZnO Particles.Polymers (Basel). 2017 Jul 2;9(7):263. doi: 10.3390/polym9070263. Polymers (Basel). 2017. PMID: 30970939 Free PMC article.
-
Water-Based Scalable Methods for Self-Cleaning Antibacterial ZnO-Nanostructured Surfaces.Ind Eng Chem Res. 2020 Aug 12;59(32):14323-14333. doi: 10.1021/acs.iecr.0c01998. Epub 2020 Jul 7. Ind Eng Chem Res. 2020. PMID: 32831473 Free PMC article.
-
A Review of Abdominal Meshes for Hernia Repair-Current Status and Emerging Solutions.Materials (Basel). 2023 Nov 10;16(22):7124. doi: 10.3390/ma16227124. Materials (Basel). 2023. PMID: 38005054 Free PMC article. Review.
References
-
- Prema P., Iniya P.A., Immanuel G. Microbial Mediated Synthesis, Characterization, Antibacterial and Synergistic Effect of Gold Nanoparticles Using Klebsiella Pneumoniae (MTCC-4030) RSC Adv. 2016;6:4601–4607. doi: 10.1039/C5RA23982F. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

