Facile Synthesis of Gold Nanoparticles with Alginate and Its Catalytic Activity for Reduction of 4-Nitrophenol and H₂O₂ Detection
- PMID: 28772911
- PMCID: PMC5459079
- DOI: 10.3390/ma10050557
Facile Synthesis of Gold Nanoparticles with Alginate and Its Catalytic Activity for Reduction of 4-Nitrophenol and H₂O₂ Detection
Abstract
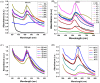
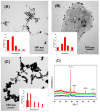
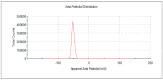
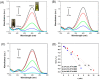
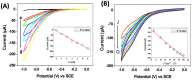
Gold nanoparticles (AuNPs) were synthesized using a facile solvothermal method with alginate sodium as both reductant and stabilizer. Formation of AuNPs was confirmed by UV-vis spectroscopic analysis. The synthesized AuNPs showed a localized surface plasmon resonance at approximately 520-560 nm. The AuNPs were characterized using transmission electron microscopy, X-ray diffraction and dynamic light scattering. Transmission electron microscopy revealed that the AuNPs were mostly nanometer-sized spherical particles. Powder X-ray diffraction analysis proved the formation of face-centered cubic structure of Au. Catalytic reduction of 4-nitrophenol was monitored via spectrophotometry using AuNPs as catalyst, and further a non-enzymatic sensor was fabricated. The results demonstrated that AuNPs presented excellent catalytic activity and provided a sensitive response to H₂O₂ detection.
Keywords: alginate; catalytic activity; gold nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Development of a novel nanoprobe from alginate functionlized gold nanoparticles and 3-(dansylamino)phenylboronic acid for glucose detection and enhanced 4-nitrophenol reduction.Carbohydr Res. 2019 Mar 1;475:11-16. doi: 10.1016/j.carres.2019.01.014. Epub 2019 Feb 7. Carbohydr Res. 2019. PMID: 30769120
-
Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au.J Hazard Mater. 2017 Jan 5;321:299-306. doi: 10.1016/j.jhazmat.2016.07.051. Epub 2016 Jul 21. J Hazard Mater. 2017. PMID: 27637096
-
Green Preparation of Ag-Au Bimetallic Nanoparticles Supported on Graphene with Alginate for Non-Enzymatic Hydrogen Peroxide Detection.Nanomaterials (Basel). 2018 Jul 8;8(7):507. doi: 10.3390/nano8070507. Nanomaterials (Basel). 2018. PMID: 29986528 Free PMC article.
-
Green Synthesis and Catalytic Activity of Gold Nanoparticles Synthesized by Artemisia capillaris Water Extract.Nanoscale Res Lett. 2016 Dec;11(1):474. doi: 10.1186/s11671-016-1694-0. Epub 2016 Oct 26. Nanoscale Res Lett. 2016. PMID: 27783375 Free PMC article.
-
Green synthesis of gold nanoparticles using fungus Mariannaea sp. HJ and their catalysis in reduction of 4-nitrophenol.Environ Sci Pollut Res Int. 2017 Sep;24(27):21649-21659. doi: 10.1007/s11356-017-9684-z. Epub 2017 Jul 27. Environ Sci Pollut Res Int. 2017. PMID: 28752308
Cited by
-
One-step green synthesis of 2D Ag-dendrite-embedded biopolymer hydrogel beads as a catalytic reactor.RSC Adv. 2021 Jun 28;11(37):22826-22834. doi: 10.1039/d1ra03536c. eCollection 2021 Jun 25. RSC Adv. 2021. PMID: 35480445 Free PMC article.
-
Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review.Bioengineering (Basel). 2019 Jun 10;6(2):53. doi: 10.3390/bioengineering6020053. Bioengineering (Basel). 2019. PMID: 31185667 Free PMC article. Review.
-
Antibacterial and hemocompatibility potentials of nano-gold-cored alginate preparation against anaerobic bacteria from acne vulgaris.Sci Rep. 2024 Mar 24;14(1):6984. doi: 10.1038/s41598-024-57643-5. Sci Rep. 2024. PMID: 38523189 Free PMC article.
-
Polydopamine functionalized hydrogel beads as magnetically separable antibacterial materials.RSC Adv. 2019 May 1;9(24):13444-13457. doi: 10.1039/c9ra00623k. eCollection 2019 Apr 30. RSC Adv. 2019. PMID: 35519566 Free PMC article.
-
Construction and Application of a Non-Enzyme Hydrogen Peroxide Electrochemical Sensor Based on Eucalyptus Porous Carbon.Sensors (Basel). 2018 Oct 15;18(10):3464. doi: 10.3390/s18103464. Sensors (Basel). 2018. PMID: 30326588 Free PMC article.
References
-
- Talib A., Khan M.S., Gedda G., Wu H.F. Stabilization of gold nanoparticles using natural plant gel: A greener step towards biological applications. J. Mol. Liq. 2016;220:463–467. doi: 10.1016/j.molliq.2016.03.079. - DOI
-
- Anand K., Gengan R.M., Phulukdaree A., Chuturgoon A. Agroforestry waste moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J. Ind. Eng. Chem. 2015;21:1105–1111. doi: 10.1016/j.jiec.2014.05.021. - DOI
-
- Alex S., Tian K., Teng S., Siegel G., Tiwari A. Simple and rapid green synthesis of micrometer scale single crystalline gold nanoplates using chitosan as the reducing agent. J. Cryst. Growth. 2014;406:12–17. doi: 10.1016/j.jcrysgro.2014.08.008. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

