Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration
- PMID: 28772927
- PMCID: PMC5541296
- DOI: 10.3390/ma10060568
Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration
Abstract
A significant body of research has considered collagen as a scaffold material for soft tissue regeneration. The main structural component of extra-cellular matrix (ECM), collagen's advantages over synthetic polymers are numerous. However, for applications where higher stiffness and stability are required, significant cross-linking may affect bioactivity. A carbodiimide (EDC) cross-linking route consumes carboxylate groups that are key to collagen's essential cell recognition motifs (GxOGER). Fibrinogen was considered as a promising additive as it plays a key role in the process of wound repair and contains RGD integrin binding sites which bind to a variety of cells, growth factors and cytokines. Fibrinogen's binding sites however, also contain the same carboxylate groups as collagen. We have successfully produced highly interconnected, porous collagen-fibrinogen scaffolds using a lyophilisation technique and micro-computed tomography demonstrated minimal influence of either fibrinogen content or cross-linking concentration on the scaffold structure. The specific biological effect of fibrinogen additions into cross-linked collagen are considered by using films as a model for the struts of bulk scaffolds. By considering various additions of fibrinogen to the collagen film with increasing degrees of cross-linking, this study demonstrates a significant biological advantage with fibrinogen addition across the cross-linking concentrations typically applied to collagen-based scaffolds.
Keywords: adhesion; collagen; fibrinogen; lyophilisation; micro-computed tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

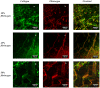
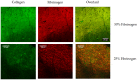



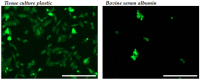


Similar articles
-
The effect of cross-linking time on a porous freeze-dried collagen scaffold using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide as a cross-linker.J Appl Biomater Biomech. 2008 May-Aug;6(2):89-94. J Appl Biomater Biomech. 2008. PMID: 20740451
-
Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds.J Mater Sci Mater Med. 2016 Jan;27(1):14. doi: 10.1007/s10856-015-5627-8. Epub 2015 Dec 16. J Mater Sci Mater Med. 2016. PMID: 26676860 Free PMC article.
-
Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering.J Mech Behav Biomed Mater. 2012 Jun;10:62-74. doi: 10.1016/j.jmbbm.2012.02.028. Epub 2012 Mar 16. J Mech Behav Biomed Mater. 2012. PMID: 22520419
-
Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives.Polymers (Basel). 2016 Feb 4;8(2):42. doi: 10.3390/polym8020042. Polymers (Basel). 2016. PMID: 30979136 Free PMC article. Review.
-
Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins.Adv Exp Med Biol. 2014;802:31-47. doi: 10.1007/978-94-007-7893-1_3. Adv Exp Med Biol. 2014. PMID: 24443019 Review.
Cited by
-
Enhanced wound healing via collagen-turnover-driven transfer of PDGF-BB gene in a murine wound model.ACS Appl Bio Mater. 2020 Jun 15;3(6):3500-3517. doi: 10.1021/acsabm.9b01147. Epub 2020 May 4. ACS Appl Bio Mater. 2020. PMID: 32656505 Free PMC article.
-
Engineered Collagen Matrices.Bioengineering (Basel). 2020 Dec 16;7(4):163. doi: 10.3390/bioengineering7040163. Bioengineering (Basel). 2020. PMID: 33339157 Free PMC article. Review.
-
Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds for infection control in wounds.Acta Biomater. 2020 Feb;103:115-128. doi: 10.1016/j.actbio.2019.12.014. Epub 2019 Dec 13. Acta Biomater. 2020. PMID: 31843720 Free PMC article.
References
-
- Ko Y.G., Kawazoe N., Tateishi T., Chen G. Preparation of novel collagen sponges using an ice particulate template. J. Bioact. Compat. Polym. 2010;25:360–373. doi: 10.1177/0883911510370002. - DOI
-
- Parenteau-Bareil R., Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. doi: 10.3390/ma3031863. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

