Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements
- PMID: 28772959
- PMCID: PMC5553423
- DOI: 10.3390/ma10060606
Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements
Abstract
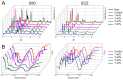
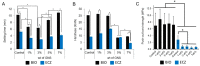
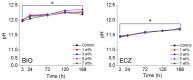
Bioactive calcium silicate cements are widely used to induce mineralization, to cement prosthetic parts, in the management of tooth perforations, and other areas. Nonetheless, they can present clinical disadvantages, such as long setting time and modest physico-mechanical properties. The objective of this work was to evaluate the potential of graphene nanosheets (GNS) to improve two bioactive cements. GNS were obtained via reduction of graphite oxide. GNS were mixed (1, 3, 5, and 7 wt %) with Biodentine (BIO) and Endocem Zr (ECZ), and the effects on setting time, hardness, push-out strength, pH profile, cell proliferation, and mineralization were evaluated. Statistics were performed with two-way ANOVA and Tukey test (α = 0.05). GNS has not interfered in the composition of the set cements as confirmed by Raman, FT-IR and XRD. GNS (1 and 3 wt %) shortened the setting time, increased hardness of both materials but decreased significantly the push-out strength of ECZ. pH was not affected but 1 wt % and 7 wt % to ECZ and 5 wt % to BIO increased the mineralization compared to the controls. In summary, GNS may be an alternative to improve the physico-mechanical properties and bioactivity of cements. Nonetheless, the use of GNS may not be advised for all materials when effective bonding is a concern.
Keywords: Biodentine; dental pulp stem cells; graphene; mineral trioxide aggregate; push-out bond strength.
Conflict of interest statement
Authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

