Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review
- PMID: 28773045
- PMCID: PMC5551730
- DOI: 10.3390/ma10070687
Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review
Abstract
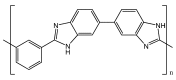
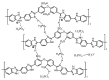
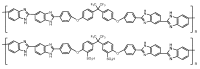
Polymer fuel cells operating above 100 °C (High Temperature Polymer Electrolyte Membrane Fuel Cells, HT-PEMFCs) have gained large interest for their application to automobiles. The HT-PEMFC devices are typically made of membranes with poly(benzimidazoles), although other polymers, such as sulphonated poly(ether ether ketones) and pyridine-based materials have been reported. In this critical review, we address the state-of-the-art of membrane fabrication and their properties. A large number of papers of uneven quality has appeared in the literature during the last few years, so this review is limited to works that are judged as significant. Emphasis is put on proton transport and the physico-chemical mechanisms of proton conductivity.
Keywords: PBI; fuel cells; high temperature; membrane; polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- EG&G Technical Services, Inc. Fuel Cell Handbook. 7th ed. U.S. Department of Energy; Morgantown, WV, USA: 2004.
-
- Araya S.S., Zhou F., Liso V., Sahlin S.L., Vang J.R., Thomas S., Gao X., Jeppesen C., Kær S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy. 2016;41:21310–21344. doi: 10.1016/j.ijhydene.2016.09.024. - DOI
-
- Liu Y., Lehnert W., Janßen H., Samsun R.C., Stolten D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources. 2016;311:91–102. doi: 10.1016/j.jpowsour.2016.02.033. - DOI
-
- Wang C., Wang S., Peng L., Zhang J., Shao Z., Huang J., Sun C., Ouyang M., He X. Recent progress on the key materials and components for proton exchange membrane fuel cells in vehicle applications. Energies. 2016;9:603. doi: 10.3390/en9080603. - DOI
-
- Rosli R.E., Sulong A.B., Daud W.R.W., Zulkifley M.A., Husaini T., Rosli M.I., Majlan E.H., Haque M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy. 2017;42:9293–9314. doi: 10.1016/j.ijhydene.2016.06.211. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

