Study of Superbase-Based Deep Eutectic Solvents as the Catalyst in the Chemical Fixation of CO₂ into Cyclic Carbonates under Mild Conditions
- PMID: 28773128
- PMCID: PMC5551802
- DOI: 10.3390/ma10070759
Study of Superbase-Based Deep Eutectic Solvents as the Catalyst in the Chemical Fixation of CO₂ into Cyclic Carbonates under Mild Conditions
Abstract
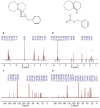
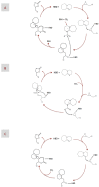
Superbases have shown high performance as catalysts in the chemical fixation of CO₂ to epoxides. The proposed reaction mechanism typically assumes the formation of a superbase, the CO₂ adduct as the intermediate, most likely because of the well-known affinity between superbases and CO₂, i.e., superbases have actually proven quite effective for CO₂ absorption. In this latter use, concerns about the chemical stability upon successive absorption-desorption cycles also merits attention when using superbases as catalysts. In this work, ¹H NMR spectroscopy was used to get further insights about (1) whether a superbase, the CO₂ adduct, is formed as an intermediate and (2) the chemical stability of the catalyst after reaction. For this purpose, we proposed as a model system the chemical fixation of CO₂ to epichlorohydrin (EP) using a deep eutectic solvent (DES) composed of a superbase, e.g., 2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine (TBD) or 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine (DBU), as a hydrogen acceptor and an alcohol as a hydrogen bond donor, e.g., benzyl alcohol (BA), ethylene glycol (EG), and methyldiethanolamine (MDEA), as the catalyst. The resulting carbonate was obtained with yields above 90% and selectivities approaching 100% after only two hours of reaction in pseudo-mild reaction conditions, e.g., 1.2 bars and 100 °C, and after 20 h if the reaction conditions of choice were even milder, e.g., 1.2 bars and 50 °C. These results were in agreement with previous works using bifunctional catalytic systems composed of a superbase and a hydrogen bond donor (HBD) also reporting good yields and selectivities, thus confirming the suitability of our choice to perform this study.
Keywords: CO2 absorption; CO2 fixation; eutectic solvents; superbases.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
Figures





References
-
- Williams C.K., Hillmyer M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008;48:1–10. doi: 10.1080/15583720701834133. - DOI
-
- Yang Z.-Z., Zhao Y.-N., He L.-N. CO2 chemistry: Task-specific ionic liquids for CO2 capture/activation and subsequent conversión. RSC Adv. 2011;1:545–567. doi: 10.1039/c1ra00307k. - DOI
-
- Otto A., Grube T., Schiebahn S., Stolten D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015;8:3283–3297. doi: 10.1039/C5EE02591E. - DOI
-
- International Energy Agency. [(accessed on 13 March 2015)]; Available online: http://www.iea.org/newsroomandevents/news/2015/march/global-energy-relat....
-
- Andersson A.M., Abraham D.P., Haasch R., MacLaren S., Liu J., Amine K. Surface characterization of electrodes from high power lithium-ion batteries. J. Electrochem. Soc. 2002;149:A1358–A1369. doi: 10.1149/1.1505636. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

