Nanostructured ZnO in a Metglas/ZnO/Hemoglobin Modified Electrode to Detect the Oxidation of the Hemoglobin Simultaneously by Cyclic Voltammetry and Magnetoelastic Resonance
- PMID: 28773209
- PMCID: PMC5578215
- DOI: 10.3390/ma10080849
Nanostructured ZnO in a Metglas/ZnO/Hemoglobin Modified Electrode to Detect the Oxidation of the Hemoglobin Simultaneously by Cyclic Voltammetry and Magnetoelastic Resonance
Abstract
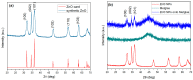
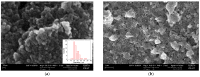
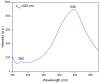
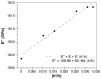
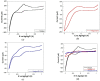
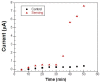
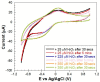
In the present work, a nanostructured ZnO layer was synthesized onto a Metglas magnetoelastic ribbon to immobilize hemoglobin (Hb) on it and study the Hb's electrochemical behavior towards hydrogen peroxide. Hb oxidation by H₂O₂ was monitored simultaneously by two different techniques: Cyclic Voltammetry (CV) and Magnetoelastic Resonance (MR). The Metglas/ZnO/Hb system was simultaneously used as a working electrode for the CV scans and as a magnetoelastic sensor excited by external coils, which drive it to resonance and interrogate it. The ZnO nanoparticles for the ZnO layer were grown hydrothermally and fully characterized by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and photoluminescence (PL). Additionally, the ZnO layer's elastic modulus was measured using a new method, which makes use of the Metglas substrate. For the detection experiments, the electrochemical cell was performed with a glass vial, where the three electrodes (working, counter and reference) were immersed into PBS (Phosphate Buffer Solution) solution and small H₂O₂ drops were added, one at a time. CV scans were taken every 30 s and 5 min after the addition of each drop and meanwhile a magnetoelastic measurement was taken by the external coils. The CV plots reveal direct electrochemical behavior of Hb and display good electrocatalytic response to the reduction of H₂O₂. The measured catalysis currents increase linearly with the H₂O₂ concentration in a wide range of 25-350 μM with a correlation coefficient 0.99. The detection limit is 25-50 μM. Moreover, the Metglas/ZnO/Hb electrode displays rapid response (30 s) to H₂O₂, and exhibits good stability and reproducibility of the measurements. On the other hand, the magnetoelastic measurements show a small linear mass increase versus the H₂O₂ concentration with a slope of 152 ng/μM, which is probably due to H₂O₂ adsorption in ZnO during the electrochemical reaction. No such effects were detected during the control experiment when only PBS solution was present for a long time.
Keywords: Hemoglobin; Metglas; ZnO nanostructures; characterizations; magnetoelastic resonance; sensors; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Duan G., Li Y., Wen Y., Ma X., Wang Y., Ji J., Wu P., Zhang Z., Yang H. Direct electrochemistry and electrocatalysis of Hemoglobin/ZnO-Chitosan/nano-Au modified glassy carbon electrode. Electroanalysis. 2008;20:2454–2459. doi: 10.1002/elan.200804337. - DOI
-
- Jing Z.H., Zhan J.H. Fabrication and gas sensing properties of porous ZnO nanoplates. Adv. Mater. 2008;20:4547–4551. doi: 10.1002/adma.200800243. - DOI
-
- Fang X., Bando Y., Liao M., Gautam U.K., Zhi C., Dierre B., Liu B., Zhai T., Sekiguchi T., Koide Y., et al. Single-crystalline ZnS nanobelts as ultraviolet-light sensors. Adv. Mater. 2009;21:2034–2039. doi: 10.1002/adma.200802441. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

