Er-Doped LiNi0.5Mn1.5O₄ Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries
- PMID: 28773224
- PMCID: PMC5578225
- DOI: 10.3390/ma10080859
Er-Doped LiNi0.5Mn1.5O₄ Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries
Abstract
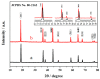
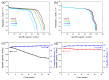
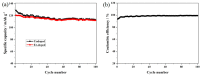
The Er-doped LiNi0.5Mn1.5O₄ (LiNi0.495Mn1.495Er0.01O₄) sample was successfully prepared by citric acid-assisted sol-gel method with erbium oxide as an erbium source for the first time. Compared with the undoped sample, the Er-doped LiNi0.5Mn1.5O₄ sample maintained the basic spinel structure, suggesting that the substitution of Er3+ ions for partial nickel and manganese ions did not change the intrinsic structure of LiNi0.5Mn1.5O₄. Moreover, the Er-doped LiNi0.5Mn1.5O₄ sample showed better size distribution and regular octahedral morphology. Electrochemical measurements indicated that the Er-doping could have a positive impact on the electrochemical properties. When cycled at 0.5 C, the Er-doped LiNi0.5Mn1.5O₄ sample exhibited an initial discharge capacity of 120.6 mAh·g-1, and the capacity retention of this sample reached up to 92.9% after 100 cycles. As the charge/discharge rate restored from 2.0 C to 0.2 C, the discharge capacity of this sample still exhibited 123.7 mAh·g-1 with excellent recovery rate. Since the bonding energy of Er-O (615 kJ·mol-1) was higher than that of Mn-O (402 kJ·mol -1) and Ni-O (392 kJ·mol-1), these outstanding performance could be attributed to the increased structure stability as well as the reduced aggregation behavior and small charge transfer resistance of the Er-doped LiNi0.5Mn1.5O₄.
Keywords: Er-doping; LiNi0.5Mn1.5O4; Lithium-ion battery; citric acid-assisted sol-gel method; cycling stability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Facile synthesis of hierarchical micro/nanostructured MnO material and its excellent lithium storage property and high performance as anode in a MnO/LiNi0.5Mn1.5O(4-δ) lithium ion battery.ACS Appl Mater Interfaces. 2013 Jul 10;5(13):6316-23. doi: 10.1021/am401355w. Epub 2013 Jun 24. ACS Appl Mater Interfaces. 2013. PMID: 23758592
-
Facile synthesis and characterization of a SnO2-modified LiNi0.5Mn1.5O4 high-voltage cathode material with superior electrochemical performance for lithium ion batteries.Phys Chem Chem Phys. 2017 Apr 12;19(15):9983-9991. doi: 10.1039/c7cp00943g. Phys Chem Chem Phys. 2017. PMID: 28362012
-
Synthesis and Electrochemical Properties of LiNi0.5Mn1.5O4 Cathode Materials with Cr3+ and F- Composite Doping for Lithium-Ion Batteries.Nanoscale Res Lett. 2017 Dec;12(1):414. doi: 10.1186/s11671-017-2172-z. Epub 2017 Jun 15. Nanoscale Res Lett. 2017. PMID: 28622717 Free PMC article.
-
Research Progress in Improving the Cycling Stability of High-Voltage LiNi0.5Mn1.5O4 Cathode in Lithium-Ion Battery.Nanomicro Lett. 2017;9(2):22. doi: 10.1007/s40820-016-0123-3. Epub 2017 Jan 4. Nanomicro Lett. 2017. PMID: 30460318 Free PMC article. Review.
-
Microwaves in clean energy technologies.Philos Trans A Math Phys Eng Sci. 2025 May 22;383(2297):20240394. doi: 10.1098/rsta.2024.0394. Epub 2025 May 22. Philos Trans A Math Phys Eng Sci. 2025. PMID: 40400321 Free PMC article. Review.
Cited by
-
Enhanced Cycling Stability of LiCuxMn1.95-xSi0.05O₄ Cathode Material Obtained by Solid-State Method.Materials (Basel). 2018 Jul 27;11(8):1302. doi: 10.3390/ma11081302. Materials (Basel). 2018. PMID: 30060499 Free PMC article.
-
The Effects of Ru4+ Doping on LiNi0.5Mn1.5O4 with Two Crystal Structures.Materials (Basel). 2022 Jun 16;15(12):4273. doi: 10.3390/ma15124273. Materials (Basel). 2022. PMID: 35744330 Free PMC article.
-
Improved Electrochemical Properties of LiMn2O4-Based Cathode Material Co-Modified by Mg-Doping and Octahedral Morphology.Materials (Basel). 2019 Aug 31;12(17):2807. doi: 10.3390/ma12172807. Materials (Basel). 2019. PMID: 31480434 Free PMC article.
-
Utilizing the Intrinsic Thermal Instability of Swedenborgite Structured YBaCo4O7+δ as an Opportunity for Material Engineering in Lithium-Ion Batteries by Er and Ga Co-Doping Processes.Materials (Basel). 2021 Aug 14;14(16):4565. doi: 10.3390/ma14164565. Materials (Basel). 2021. PMID: 34443088 Free PMC article.
References
-
- Fergus J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources. 2010;195:939–954. doi: 10.1016/j.jpowsour.2009.08.089. - DOI
-
- Cheng J., Li X., Wang Z., Guo H. Hydrothermal synthesis of LiNi0.5Mn1.5O4 sphere and its performance as high-voltage cathode material for lithium ion batteries. Ceram. Int. 2016;42:3715–3719. doi: 10.1016/j.ceramint.2015.11.031. - DOI
-
- Sulochana A., Thirunakaran R., Sivashanmugam A., Gopukumar S., Yamkiet J. Sol-gel synthesis of 5 V LiCuxMn2−xO4 as a cathode material for lithium rechargeable batteries. J. Electrochem. Soc. 2008;155:A206–A210. doi: 10.1149/1.2828030. - DOI
-
- Wei Y.J., Yan L.Y., Wang C.Z., Xu X.G., Wu F., Chen G. Effect of Ni doping on [MnO6] octahedron in LiMn2O4. J. Phys. Chem. B. 2004;108:18547–18551. doi: 10.1021/jp0479522. - DOI
-
- Shigemura H., Sakaebe H., Kageyama H., Kobayashi H., West A.R., Kanno R., Morimoto S., Nasu S., Tabuchi M. Structure and electrochemical properties of LiFexMn2−xO4 (0 ≤ x ≤ 0.5) spinel as 5 V electrode material for lithium batteries. J. Electrochem. Soc. 2001;148:A730–A736. doi: 10.1149/1.1377593. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

