Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications
- PMID: 28773266
- PMCID: PMC5456731
- DOI: 10.3390/ma9030137
Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications
Abstract
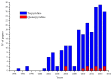
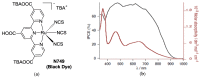
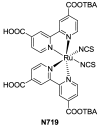
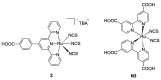
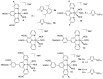
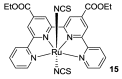
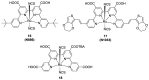
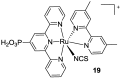
Terpyridine and quaterpyridine-based complexes allow wide light harvesting of the solar spectrum. Terpyridines, with respect to bipyridines, allow for achieving metal-complexes with lower band gaps in the metal-to-ligand transition (MLCT), thus providing a better absorption at lower energy wavelengths resulting in an enhancement of the solar light-harvesting ability. Despite the wider absorption of the first tricarboxylate terpyridyl ligand-based complex, Black Dye (BD), dye-sensitized solar cell (DSC) performances are lower if compared with N719 or other optimized bipyridine-based complexes. To further improve BD performances several modifications have been carried out in recent years affecting each component of the complexes: terpyridines have been replaced by quaterpyridines; other metals were used instead of ruthenium, and thiocyanates have been replaced by different pinchers in order to achieve cyclometalated or heteroleptic complexes. The review provides a summary on design strategies, main synthetic routes, optical and photovoltaic properties of terpyridine and quaterpyridine ligands applied to photovoltaic, and focuses on n-type DSCs.
Keywords: Ru(II) complexes; dye-sensitized solar cells; polypyridines; quaterpyridines; terpyridines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

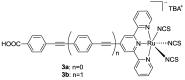
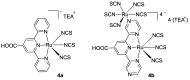
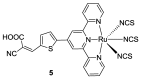
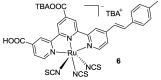


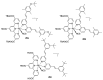
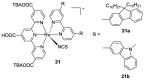
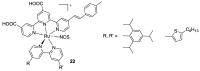
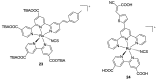
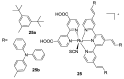

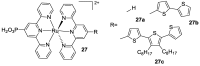
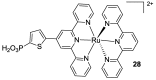
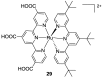
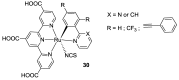

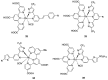
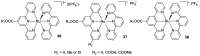
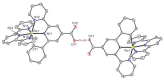

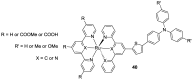
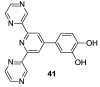
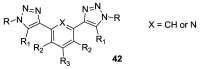
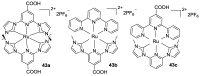
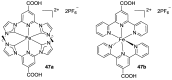
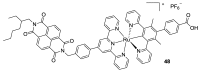
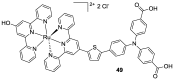
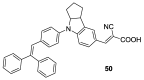
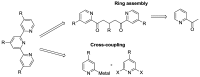
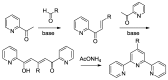

References
-
- O'Regan B., Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353:737–740. doi: 10.1038/353737a0. - DOI
-
- Barbero N., Sauvage F. Low Cost Electricity Production From Sunlight: Third-Generation Photovoltaics and the Dye-Sensitized Solar Cell. In: Moya X., Munoz-Rojas D., editors. Materials for Sustainable Energy Applications: Conversion, Storage, Transmission and Consumption. CRC Press; Boca Raton, FL, USA: 2016. pp. 87–147.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

