Polystyrene -co- Divinylbenzene PolyHIPE Monoliths in 1.0 mm Column Formats for Liquid Chromatography
- PMID: 28773337
- PMCID: PMC5456711
- DOI: 10.3390/ma9030212
Polystyrene -co- Divinylbenzene PolyHIPE Monoliths in 1.0 mm Column Formats for Liquid Chromatography
Abstract
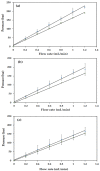
The reversed phase liquid chromatographic (RP-HPLC) separation of small molecules using a polystyrene-co-divinylbenzene (PS-co-DVB) polyHIPE stationary phases housed within 1.0 mm i.d. silcosteel columns is presented within this study. A 90% PS-co-DVB polyHIPE was covalently attached to the walls of the column housing by prior wall modification with 3-(trimethoxysilyl) propyl methacrylate and could withstand operating backpressures in excess of 200 bar at a flow rate of 1.2 mL/min. Permeability studies revealed that the monolith swelled slightly in 100% acetonitrile relative to 100% water but could nevertheless be used to separate five alkylbenzenes using a flow rate of 40 µL/min (linear velocity: 0.57 mm/s). Remarkable column-to-column reproducibility is shown with retention factor variation between 2.6% and 6.1% for two separately prepared columns.
Keywords: isocratic separation; microbore; polyHIPE; polystyrene; reversed phase LC; silcosteel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

