A Hydrogel Model Incorporating 3D-Plotted Hydroxyapatite for Osteochondral Tissue Engineering
- PMID: 28773410
- PMCID: PMC5502978
- DOI: 10.3390/ma9040285
A Hydrogel Model Incorporating 3D-Plotted Hydroxyapatite for Osteochondral Tissue Engineering
Abstract
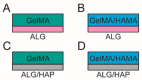
The concept of biphasic or multi-layered compound scaffolds has been explored within numerous studies in the context of cartilage and osteochondral regeneration. To date, no system has been identified that stands out in terms of superior chondrogenesis, osteogenesis or the formation of a zone of calcified cartilage (ZCC). Herein we present a 3D plotted scaffold, comprising an alginate and hydroxyapatite paste, cast within a photocrosslinkable hydrogel made of gelatin methacrylamide (GelMA), or GelMA with hyaluronic acid methacrylate (HAMA). We hypothesized that this combination of 3D plotting and hydrogel crosslinking would form a high fidelity, cell supporting structure that would allow localization of hydroxyapatite to the deepest regions of the structure whilst taking advantage of hydrogel photocrosslinking. We assessed this preliminary design in terms of chondrogenesis in culture with human articular chondrocytes, and verified whether the inclusion of hydroxyapatite in the form presented had any influence on the formation of the ZCC. Whilst the inclusion of HAMA resulted in a better chondrogenic outcome, the effect of HAP was limited. We overall demonstrated that formation of such compound structures is possible, providing a foundation for future work. The development of cohesive biphasic systems is highly relevant for current and future cartilage tissue engineering.
Keywords: 3D plotting; alginate; cartilage tissue engineering; chondrocyte; chondrogenesis; gelatin; hydrogel; hydroxyapatite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Athanasiou K.A., Darling E.M., DuRaine G.D., Hu J.C., Reddi A.H. Articular Cartilage. CRC Press, Taylor & Francis Group; Boca Raton, FL, USA: 2013. p. 425.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

