Structural Interpretation of the Large Slowdown of Water Dynamics at Stacked Phospholipid Membranes for Decreasing Hydration Level: All-Atom Molecular Dynamics
- PMID: 28773441
- PMCID: PMC5503093
- DOI: 10.3390/ma9050319
Structural Interpretation of the Large Slowdown of Water Dynamics at Stacked Phospholipid Membranes for Decreasing Hydration Level: All-Atom Molecular Dynamics
Abstract
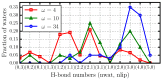
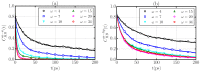
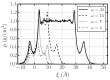
Hydration water determines the stability and function of phospholipid membranes as well as the interaction of membranes with other molecules. Experiments and simulations have shown that water dynamics slows down dramatically as the hydration decreases, suggesting that the interfacial water that dominates the average dynamics at low hydration is slower than water away from the membrane. Here, based on all-atom molecular dynamics simulations, we provide an interpretation of the slowdown of interfacial water in terms of the structure and dynamics of water-water and water-lipid hydrogen bonds (HBs). We calculate the rotational and translational slowdown of the dynamics of water confined in stacked phospholipid membranes at different levels of hydration, from completely hydrated to poorly hydrated membranes. For all hydrations, we analyze the distribution of HBs and find that water-lipids HBs last longer than water-water HBs and that at low hydration most of the water is in the interior of the membrane. We also show that water-water HBs become more persistent as the hydration is lowered. We attribute this effect (i) to HBs between water molecules that form, in turn, persistent HBs with lipids; (ii) to the hindering of the H-bonding switching between water molecules due to the lower water density at the interface; and (iii) to the higher probability of water-lipid HBs as the hydration decreases. Our interpretation of the large dynamic slowdown in water under dehydration is potentially relevant in understanding membrane biophysics at different hydration levels.
Keywords: confinement; diffusion; molecular dynamics; phospholipid membrane; water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Insights on Hydrogen Bond Network of Water in Phospholipid Membranes: An Infrared Study at Varying Hydration.Membranes (Basel). 2025 Feb 4;15(2):46. doi: 10.3390/membranes15020046. Membranes (Basel). 2025. PMID: 39997672 Free PMC article.
-
Insights into hydrogen bond dynamics at the interface of the charged monolayer-protected Au nanoparticle from molecular dynamics simulation.J Chem Phys. 2013 May 14;138(18):184703. doi: 10.1063/1.4803504. J Chem Phys. 2013. PMID: 23676060
-
Hydration and Nanoconfined Water: Insights from Computer Simulations.Subcell Biochem. 2015;71:161-87. doi: 10.1007/978-3-319-19060-0_7. Subcell Biochem. 2015. PMID: 26438265 Review.
-
Structural and functional properties of hydration and confined water in membrane interfaces.Biochim Biophys Acta. 2008 Dec;1778(12):2655-70. doi: 10.1016/j.bbamem.2008.08.025. Epub 2008 Sep 12. Biochim Biophys Acta. 2008. PMID: 18834854 Review.
-
Molecular modeling and simulation of water near model micelles: diffusion, rotational relaxation and structure at the hydration interface.J Phys Chem B. 2006 Jun 15;110(23):11504-10. doi: 10.1021/jp057282f. J Phys Chem B. 2006. PMID: 16771426
Cited by
-
Broadband dielectric spectroscopy from sub GHz to THz of hydrated lipid bilayer of DMPC.Eur Phys J E Soft Matter. 2019 Oct 30;42(10):139. doi: 10.1140/epje/i2019-11901-1. Eur Phys J E Soft Matter. 2019. PMID: 31664606
-
Water populations in restricted environments of lipid membrane interphases.Eur Phys J E Soft Matter. 2016 Oct;39(10):94. doi: 10.1140/epje/i2016-16094-5. Epub 2016 Oct 21. Eur Phys J E Soft Matter. 2016. PMID: 27761781
-
Hydrophobic Homopolymer's Coil-Globule Transition and Adsorption onto a Hydrophobic Surface under Different Conditions.J Phys Chem B. 2023 Jun 29;127(25):5541-5552. doi: 10.1021/acs.jpcb.3c00937. Epub 2023 Jun 19. J Phys Chem B. 2023. PMID: 37334684 Free PMC article.
-
Insights on Hydrogen Bond Network of Water in Phospholipid Membranes: An Infrared Study at Varying Hydration.Membranes (Basel). 2025 Feb 4;15(2):46. doi: 10.3390/membranes15020046. Membranes (Basel). 2025. PMID: 39997672 Free PMC article.
-
Hydration Layer of Only a Few Molecules Controls Lipid Mobility in Biomimetic Membranes.J Am Chem Soc. 2021 Sep 15;143(36):14551-14562. doi: 10.1021/jacs.1c04314. Epub 2021 Aug 3. J Am Chem Soc. 2021. PMID: 34342967 Free PMC article.
References
-
- Hamley I.W. Introduction to Soft Matter. John Wiley and Sons; West Sussex, UK: 2007.
-
- Fitter J., Lechner R.E., Dencher N.A. Interactions of hydration water and biological membranes studied by neutron scattering. J. Phys. Chem. B. 1999;103:8036–8050. doi: 10.1021/jp9912410. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

