Diketonylpyridinium Cations as a Support of New Ionic Liquid Crystals and Ion-Conductive Materials: Analysis of Counter-Ion Effects
- PMID: 28773485
- PMCID: PMC5503038
- DOI: 10.3390/ma9050360
Diketonylpyridinium Cations as a Support of New Ionic Liquid Crystals and Ion-Conductive Materials: Analysis of Counter-Ion Effects
Abstract
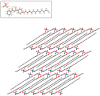
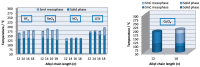
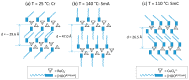
Ionic liquid crystals (ILCs) allow the combination of the high ionic conductivity of ionic liquids (ILs) with the supramolecular organization of liquid crystals (LCs). ILCs salts were obtained by the assembly of long-chained diketonylpyridinium cations of the type [HOOR(n)pyH]⁺ and BF₄-, ReO₄-, NO₃-, CF₃SO₃-, CuCl₄2- counter-ions. We have studied the thermal behavior of five series of compounds by differential scanning calorimetry (DSC) and hot stage polarized light optical microscopy (POM). All materials show thermotropic mesomorphism as well as crystalline polymorphism. X-ray diffraction of the [HOOR(12)pyH][ReO₄] crystal reveals a layered structure with alternating polar and apolar sublayers. The mesophases also exhibit a lamellar arrangement detected by variable temperature powder X-ray diffraction. The CuCl₄2- salts exhibit the best LC properties followed by the ReO₄- ones due to low melting temperature and wide range of existence. The conductivity was probed for the mesophases in one species each from the ReO₄-, and CuCl₄2- families, and for the solid phase in one of the non-mesomorphic Cl- salts. The highest ionic conductivity was found for the smectic mesophase of the ReO₄- containing salt, whereas the solid phases of all salts were dominated by electronic contributions. The ionic conductivity may be favored by the mesophase lamellar structure.
Keywords: ionic conductivity; ionic liquid crystals; ionic salts; liquid crystals; polymorphism; smectic mesophase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
New Pyrazolium Salts as a Support for Ionic Liquid Crystals and Ionic Conductors.Materials (Basel). 2018 Apr 3;11(4):548. doi: 10.3390/ma11040548. Materials (Basel). 2018. PMID: 29614030 Free PMC article.
-
Dicyanamide Salts that Adopt Smectic, Columnar, or Bicontinuous Cubic Liquid-Crystalline Mesophases.Chemistry. 2018 Apr 25;24(24):6399-6411. doi: 10.1002/chem.201705794. Epub 2018 Apr 14. Chemistry. 2018. PMID: 29446859
-
Designer ionic liquid crystals based on congruently shaped guanidinium sulfonates.Chemistry. 2012 Mar 5;18(10):3014-22. doi: 10.1002/chem.201101925. Epub 2012 Feb 1. Chemistry. 2012. PMID: 22298322
-
Ionic Liquid Crystals as Chromogenic Materials.Materials (Basel). 2024 Sep 17;17(18):4563. doi: 10.3390/ma17184563. Materials (Basel). 2024. PMID: 39336305 Free PMC article. Review.
-
Amino Acid and Peptide-Based Liquid Crystals: An Overview.Curr Org Synth. 2021;18(4):333-351. doi: 10.2174/1570179417666200916092109. Curr Org Synth. 2021. PMID: 32938353 Review.
References
-
- Gordon C.M. New developments in catalysis using ionic liquids. Appl. Cat. A Gen. 2001;222:101–117. doi: 10.1016/S0926-860X(01)00834-1. - DOI
-
- Wasserscheid P. Ionic Liquids in Synthesis. Wiley-VCH; Weinheim, Germany: 2003.
LinkOut - more resources
Full Text Sources
Other Literature Sources

