Methacrylate Polymer Monoliths for Separation Applications
- PMID: 28773570
- PMCID: PMC5456823
- DOI: 10.3390/ma9060446
Methacrylate Polymer Monoliths for Separation Applications
Abstract
This review summarizes the development of methacrylate-based polymer monoliths for separation science applications. An introduction to monoliths is presented, followed by the preparation methods and characteristics specific to methacrylate monoliths. Both traditional chemical based syntheses and emerging additive manufacturing methods are presented along with an analysis of the different types of functional groups, which have been utilized with methacrylate monoliths. The role of methacrylate based porous materials in separation science in industrially important chemical and biological separations are discussed, with particular attention given to the most recent developments and challenges associated with these materials. While these monoliths have been shown to be useful for a wide variety of applications, there is still scope for exerting better control over the porous architectures and chemistries obtained from the different fabrication routes. Conclusions regarding this previous work are drawn and an outlook towards future challenges and potential developments in this vibrant research area are presented. Discussed in particular are the potential of additive manufacturing for the preparation of monolithic structures with pre-defined multi-scale porous morphologies and for the optimization of surface reactive chemistries.
Keywords: additive manufacturing; chromatography; methacrylate; microfluidics; monoliths; porous materials; stationary phase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


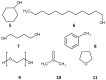








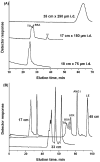
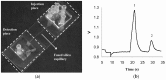


References
-
- Rouquerol J., Avnir D., Fairbridge C.W., Everett D.H., Haynes J.H., Pernicone N., Ramsay J.D.F., Sing K.S.W., Unger K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994;66:1739–1758. doi: 10.1351/pac199466081739. - DOI
-
- Synge R.L.M., Mould D.L. Electrokinetic Ultrafiltration Analysis of Polysaccharides. Analyst. 1952;77:964–969.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

