Immobilization of Magnetic Nanoparticles onto Amine-Modified Nano-Silica Gel for Copper Ions Remediation
- PMID: 28773583
- PMCID: PMC5456743
- DOI: 10.3390/ma9060460
Immobilization of Magnetic Nanoparticles onto Amine-Modified Nano-Silica Gel for Copper Ions Remediation
Abstract
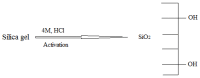
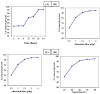
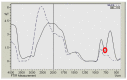
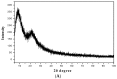
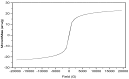
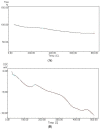
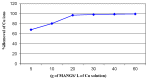
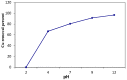
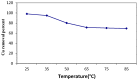
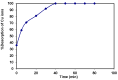
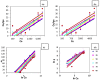
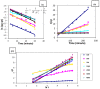
A novel nano-hybrid was synthesized through immobilization of amine-functionalized silica gel nanoparticles with nanomagnetite via a co-precipitation technique. The parameters, such as reagent concentrations, reaction temperature and time, were optimized to accomplish the nano-silica gel chelating matrix. The most proper amine-modified silica gel nanoparticles were immobilized with magnetic nanoparticles. The synthesized magnetic amine nano-silica gel (MANSG) was established and characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), Fourier transform infrared (FTIR), thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC) and vibrating sample magnetometry (VSM). The feasibility of MANSG for copper ions' remediation from wastewater was examined. MANSG achieves a 98% copper decontamination from polluted water within 90 min. Equilibrium sorption of copper ions onto MANSG nanoparticles obeyed the Langmuir equation compared to the Freundlich, Temkin, Elovich and Dubinin-Radushkevich (D-R) equilibrium isotherm models. The pseudo-second-order rate kinetics is appropriate to describe the copper sorption process onto the fabricated MANSG.
Keywords: amine-functionalized silica gel; copper remediation; equilibrium and kinetics modelling; nano-magnetic silica gel hybrid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

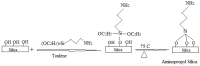





References
-
- Caroli C., Alimonti A., Petrucci F., Horvth Z. Determination of trace elements in analytical-reagent grade sodium salts by atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry after preconcentration by column solid phase extraction. Anal. Chim. Acta. 1991;360:241–245. doi: 10.1016/S0003-2670(00)80891-0. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

