Negative Thermal Expansion in Ba0.5Sr0.5Zn₂SiGeO₇
- PMID: 28773746
- PMCID: PMC5509077
- DOI: 10.3390/ma9080631
Negative Thermal Expansion in Ba0.5Sr0.5Zn₂SiGeO₇
Abstract
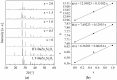
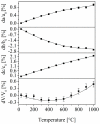
Solid solutions with the composition Ba0.5Sr0.5Zn₂Si2-xGexO₇ and BaZn₂Si2-xGexO₇ were prepared with different values of x using a conventional mixed oxide route. Both compounds exhibit very different thermal expansion, which is due to the different crystal structures. Ba0.5Sr0.5Zn₂Si2-xGexO₇ solid solutions exhibit the structure of high-temperature BaZn₂Si₂O₇ and show negative thermal expansion, which was proven via high-temperature X-ray diffraction. Up to around x = 1, the crystal structure remains the same. Above this value, the low-temperature phase becomes stable. The Sr-free solid solutions have the crystal structure of low-temperature BaZn₂Si₂O₇ and show also a limited solubility of Ge. These Sr-free compositions show transitions of low- to high-temperature phases, which are shifted to higher temperatures with increasing Ge-concentration.
Keywords: X-ray diffraction; negative thermal expansion; phase transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lin J.H., Lu G.X., Du J., Su M.Z., Loong C.-K., Richardson J.W., Jr. Phase transition and crystal structures of BaZn2Si2O7. J. Phys. Chem. Solids. 1999;60:975–983. doi: 10.1016/S0022-3697(99)00004-9. - DOI
-
- Kerstan M., Thieme C., Grosch M., Müller M., Rüssel C. BaZn2Si2O7 and the solid solution series BaZn2−xCoxSi2O7 (0 ≤ x ≤ 2) as high temperature seals for solid oxide fuel cells studied by high-temperature X-ray diffraction and dilatometry. J. Solid State Chem. 2013;207:55–60. doi: 10.1016/j.jssc.2013.09.003. - DOI
-
- Liu H., Luo W., Lin C., Du X., Yang H., Tang D., Zhang T. Reducing the reaction between boron-containing sealing glass-ceramics and lanthanum-containing cathode: Effect of La2O3. J. Eur. Ceram. Soc. 2016;36:1103–1107. doi: 10.1016/j.jeurceramsoc.2015.11.026. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

