Inkjet-Printed Organic Transistors Based on Organic Semiconductor/Insulating Polymer Blends
- PMID: 28773772
- PMCID: PMC5509261
- DOI: 10.3390/ma9080650
Inkjet-Printed Organic Transistors Based on Organic Semiconductor/Insulating Polymer Blends
Abstract
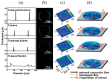
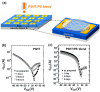
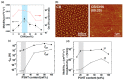
Recent advances in inkjet-printed organic field-effect transistors (OFETs) based on organic semiconductor/insulating polymer blends are reviewed in this article. Organic semiconductor/insulating polymer blends are attractive ink candidates for enhancing the jetting properties, inducing uniform film morphologies, and/or controlling crystallization behaviors of organic semiconductors. Representative studies using soluble acene/insulating polymer blends as an inkjet-printed active layer in OFETs are introduced with special attention paid to the phase separation characteristics of such blended films. In addition, inkjet-printed semiconducting/insulating polymer blends for fabricating high performance printed OFETs are reviewed.
Keywords: inkjet printing; organic field-effect transistor; organic semiconductor; polymer blend; printed electronics; soluble acene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sirringhaus H. Reliability of organic field-effect transistors. Adv. Mater. 2009;21:3859–3873. doi: 10.1002/adma.200901136. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

