Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials?
- PMID: 28774007
- PMCID: PMC5457238
- DOI: 10.3390/ma9110888
Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials?
Abstract
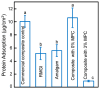
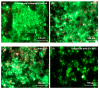
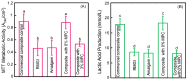
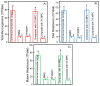
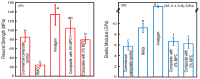
A long-time drawback of dental composites is that they accumulate more biofilms and plaques than amalgam and glass ionomer restorative materials. It would be highly desirable to develop a new composite with reduced biofilm growth, while avoiding the non-esthetics of amalgam and low strength of glass ionomer. The objectives of this study were to: (1) develop a protein-repellent composite with reduced biofilms matching amalgam and glass ionomer for the first time; and (2) investigate their protein adsorption, biofilms, and mechanical properties. Five materials were tested: A new composite containing 3% of protein-repellent 2-methacryloyloxyethyl phosphorylcholine (MPC); the composite with 0% MPC as control; commercial composite control; dental amalgam; resin-modified glass ionomer (RMGI). A dental plaque microcosm biofilm model with human saliva as inoculum was used to investigate metabolic activity, colony-forming units (CFU), and lactic acid production. Composite with 3% MPC had flexural strength similar to those with 0% MPC and commercial composite control (p > 0.1), and much greater than RMGI (p < 0.05). Composite with 3% MPC had protein adsorption that was only 1/10 that of control composites (p < 0.05). Composite with 3% MPC had biofilm CFU and lactic acid much lower than control composites (p < 0.05). Biofilm growth, metabolic activity and lactic acid on the new composite with 3% MPC were reduced to the low level of amalgam and RMGI (p > 0.1). In conclusion, a new protein-repellent dental resin composite reduced oral biofilm growth and acid production to the low levels of non-esthetic amalgam and RMGI for the first time. The long-held conclusion that dental composites accumulate more biofilms than amalgam and glass ionomer is no longer true. The novel composite is promising to finally overcome the major biofilm-accumulation drawback of dental composites in order to reduce biofilm acids and secondary caries.
Keywords: amalgam; caries inhibition; dental composite; glass ionomer; human saliva microcosm biofilm; protein repellant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Protein-repellent and antibacterial dental composite to inhibit biofilms and caries.J Dent. 2015 Feb;43(2):225-34. doi: 10.1016/j.jdent.2014.11.008. Epub 2014 Dec 3. J Dent. 2015. PMID: 25478889 Free PMC article.
-
Antibacterial and protein-repellent orthodontic cement to combat biofilms and white spot lesions.J Dent. 2015 Dec;43(12):1529-38. doi: 10.1016/j.jdent.2015.09.006. Epub 2015 Sep 30. J Dent. 2015. PMID: 26427311 Free PMC article.
-
Novel protein-repellent and biofilm-repellent orthodontic cement containing 2-methacryloyloxyethyl phosphorylcholine.J Biomed Mater Res B Appl Biomater. 2016 Jul;104(5):949-59. doi: 10.1002/jbm.b.33444. Epub 2015 May 13. J Biomed Mater Res B Appl Biomater. 2016. PMID: 25970092
-
Resin-modified glass ionomer cements (RM GICs) implications for use in pediatric dentistry.ASDC J Dent Child. 1997 Mar-Apr;64(2):131-4. ASDC J Dent Child. 1997. PMID: 9189004 Review.
-
Biofilm formation on dental restorative and implant materials.J Dent Res. 2010 Jul;89(7):657-65. doi: 10.1177/0022034510368644. Epub 2010 May 6. J Dent Res. 2010. PMID: 20448246 Review.
Cited by
-
Novel Antibacterial Copolymers Based on Quaternary Ammonium Urethane-Dimethacrylate Analogues and Triethylene Glycol Dimethacrylate.Int J Mol Sci. 2022 Apr 29;23(9):4954. doi: 10.3390/ijms23094954. Int J Mol Sci. 2022. PMID: 35563344 Free PMC article.
-
Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms.Int J Mol Sci. 2018 Oct 14;19(10):3157. doi: 10.3390/ijms19103157. Int J Mol Sci. 2018. PMID: 30322190 Free PMC article. Review.
-
The formation of cariogenic plaque to contemporary adhesive restorative materials: an in vitro study.Odontology. 2024 Oct;112(4):1090-1102. doi: 10.1007/s10266-024-00913-5. Epub 2024 Mar 19. Odontology. 2024. PMID: 38502470 Free PMC article.
-
Differences in Supragingival Microbiome in Patients with and without Full-Crown Prostheses.Dent J (Basel). 2022 Aug 15;10(8):152. doi: 10.3390/dj10080152. Dent J (Basel). 2022. PMID: 36005250 Free PMC article.
-
Characterization of the Mechanical Properties, Water Sorption, and Solubility of Antibacterial Copolymers of Quaternary Ammonium Urethane-Dimethacrylates and Triethylene Glycol Dimethacrylate.Materials (Basel). 2022 Aug 11;15(16):5530. doi: 10.3390/ma15165530. Materials (Basel). 2022. PMID: 36013665 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

