Additive Manufacturing of Patient-Customizable Scaffolds for Tubular Tissues Using the Melt-Drawing Method
- PMID: 28774013
- PMCID: PMC5457202
- DOI: 10.3390/ma9110893
Additive Manufacturing of Patient-Customizable Scaffolds for Tubular Tissues Using the Melt-Drawing Method
Abstract
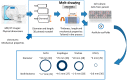
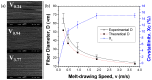
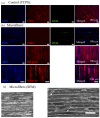
Polymeric fibrous scaffolds for guiding cell growth are designed to be potentially used for the tissue engineering (TE) of tubular organs including esophagi, blood vessels, tracheas, etc. Tubular scaffolds were fabricated via melt-drawing of highly elastic poly(l-lactide-co-ε-caprolactone) (PLC) fibers layer-by-layer on a cylindrical mandrel. The diameter and length of the scaffolds are customizable via 3D printing of the mandrel. Thickness of the scaffolds was varied by changing the number of layers of the melt-drawing process. The morphology and tensile properties of the PLC fibers were investigated. The fibers were highly aligned with a uniform diameter. Their diameters and tensile properties were tunable by varying the melt-drawing speeds. These tailorable topographies and tensile properties show that the additive-based scaffold fabrication technique is customizable at the micro- and macro-scale for different tubular tissues. The merits of these scaffolds in TE were further shown by the finding that myoblast and fibroblast cells seeded onto the scaffolds in vitro showed appropriate cell proliferation and distribution. Human mesenchymal stem cells (hMSCs) differentiated to smooth muscle lineage on the microfibrous scaffolds in the absence of soluble induction factors, showing cellular shape modulation and scaffold elasticity may encourage the myogenic differentiation of stem cells.
Keywords: additive manufacturing; melt-drawing; scaffold; tissue engineering; tubular tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Characterization, mechanical behavior and in vitro evaluation of a melt-drawn scaffold for esophageal tissue engineering.J Mech Behav Biomed Mater. 2016 Apr;57:246-59. doi: 10.1016/j.jmbbm.2015.12.015. Epub 2015 Dec 21. J Mech Behav Biomed Mater. 2016. PMID: 26735183
-
Development of an in-process UV-crosslinked, electrospun PCL/aPLA-co-TMC composite polymer for tubular tissue engineering applications.Acta Biomater. 2016 May;36:231-40. doi: 10.1016/j.actbio.2016.03.013. Epub 2016 Mar 8. Acta Biomater. 2016. PMID: 26969522
-
High Throughput Manufacturing of Bio-Resorbable Micro-Porous Scaffolds Made of Poly(L-lactide-co-ε-caprolactone) by Micro-Extrusion for Soft Tissue Engineering Applications.Polymers (Basel). 2019 Dec 24;12(1):34. doi: 10.3390/polym12010034. Polymers (Basel). 2019. PMID: 31878300 Free PMC article.
-
Hyaluronic Acid-Coated Melt Electrowritten Scaffolds Promote Myoblast Attachment, Alignment, and Differentiation.bioRxiv [Preprint]. 2025 Mar 10:2025.03.06.641880. doi: 10.1101/2025.03.06.641880. bioRxiv. 2025. PMID: 40161586 Free PMC article. Preprint.
-
Recent advances in melt electro writing for tissue engineering for 3D printing of microporous scaffolds for tissue engineering.Front Bioeng Biotechnol. 2022 Aug 17;10:896719. doi: 10.3389/fbioe.2022.896719. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36061443 Free PMC article. Review.
Cited by
-
Recent Advances in Biomaterials for 3D Printing and Tissue Engineering.J Funct Biomater. 2018 Mar 1;9(1):22. doi: 10.3390/jfb9010022. J Funct Biomater. 2018. PMID: 29494503 Free PMC article. Review.
-
Advances in 3D Printing for Tissue Engineering.Materials (Basel). 2021 Jun 8;14(12):3149. doi: 10.3390/ma14123149. Materials (Basel). 2021. PMID: 34201163 Free PMC article. Review.
-
Special Issue: 3D Printing for Biomedical Engineering.Materials (Basel). 2017 Feb 28;10(3):243. doi: 10.3390/ma10030243. Materials (Basel). 2017. PMID: 28772604 Free PMC article.
-
Investigation of Quasi-Static Indentation Response of Inkjet Printed Sandwich Structures under Various Indenter Geometries.Materials (Basel). 2017 Mar 14;10(3):290. doi: 10.3390/ma10030290. Materials (Basel). 2017. PMID: 28772649 Free PMC article.
References
-
- Hoogenkamp H.R., Koens M.J.W., Geutjes P.J., Ainoedhofer H., Wanten G., Tiemessen D.M., Hilborn J., Gupta B., Feitz W.F.J., Daamen W.F. Seamless vascularized large-diameter tubular collagen scaffolds reinforced with polymer knittings for esophageal regenerative medicine. Tissue Eng. Part C Methods. 2014;20:423–430. doi: 10.1089/ten.tec.2013.0485. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

