Fc-Binding Ligands of Immunoglobulin G: An Overview of High Affinity Proteins and Peptides
- PMID: 28774114
- PMCID: PMC5456964
- DOI: 10.3390/ma9120994
Fc-Binding Ligands of Immunoglobulin G: An Overview of High Affinity Proteins and Peptides
Abstract
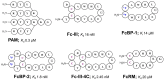
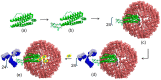
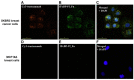
The rapidly increasing application of antibodies has inspired the development of several novel methods to isolate and target antibodies using smart biomaterials that mimic the binding of Fc-receptors to antibodies. The Fc-binding domain of antibodies is the primary binding site for e.g., effector proteins and secondary antibodies, whereas antigens bind to the Fab region. Protein A, G, and L, surface proteins expressed by pathogenic bacteria, are well known to bind immunoglobulin and have been widely exploited in antibody purification strategies. Several difficulties are encountered when bacterial proteins are used in antibody research and application. One of the major obstacles hampering the use of bacterial proteins is sample contamination with trace amounts of these proteins, which can invoke an immune response in the host. Many research groups actively develop synthetic ligands that are able to selectively and strongly bind to antibodies. Among the reported ligands, peptides that bind to the Fc-domain of antibodies are attractive tools in antibody research. Besides their use as high affinity ligands in antibody purification chromatography, Fc-binding peptides are applied e.g., to localize antibodies on nanomaterials and to increase the half-life of proteins in serum. In this review, recent developments of Fc-binding peptides are presented and their binding characteristics and diverse applications are discussed.
Keywords: Fc-binding peptide; Protein A; Protein A mimics; Protein G; Protein L; affinity column chromatography; antibody; targeted drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


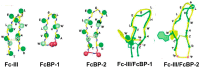



Similar articles
-
Cyclic peptide ligand with high binding capacity for affinity purification of immunoglobulin G.J Chromatogr A. 2016 Sep 30;1466:105-12. doi: 10.1016/j.chroma.2016.09.007. Epub 2016 Sep 3. J Chromatogr A. 2016. PMID: 27608618
-
Efficient selection of IgG Fc domain-binding peptides fused to fluorescent protein using E. coli expression system and dot-blotting assay.Peptides. 2010 Feb;31(2):202-6. doi: 10.1016/j.peptides.2009.12.009. Epub 2009 Dec 16. Peptides. 2010. PMID: 20025916
-
IgG Fc Affinity Ligands and Their Applications in Antibody-Involved Drug Delivery: A Brief Review.Pharmaceutics. 2023 Jan 5;15(1):187. doi: 10.3390/pharmaceutics15010187. Pharmaceutics. 2023. PMID: 36678816 Free PMC article. Review.
-
The Purification of Natural and Recombinant Peptide Antibodies by Affinity Chromatographic Strategies.Methods Mol Biol. 2015;1348:153-65. doi: 10.1007/978-1-4939-2999-3_15. Methods Mol Biol. 2015. PMID: 26424271
-
Separation of antigens and antibodies by immunoaffinity chromatography.Pharm Biol. 2012 Aug;50(8):1038-44. doi: 10.3109/13880209.2011.653493. Epub 2012 Apr 6. Pharm Biol. 2012. PMID: 22480305 Review.
Cited by
-
Nanoplasmonic Avidity-Based Detection and Quantification of IgG Aggregates.Anal Chem. 2022 Nov 15;94(45):15754-15762. doi: 10.1021/acs.analchem.2c03446. Epub 2022 Nov 1. Anal Chem. 2022. PMID: 36318700 Free PMC article.
-
Considerations for the Design of Antibody-Based Therapeutics.J Pharm Sci. 2020 Jan;109(1):74-103. doi: 10.1016/j.xphs.2019.05.031. Epub 2019 Jun 4. J Pharm Sci. 2020. PMID: 31173761 Free PMC article. Review.
-
Cleavage Under Targets and Release Using Nuclease (CUT&RUN) in Macrophages.Methods Mol Biol. 2024;2846:151-167. doi: 10.1007/978-1-0716-4071-5_10. Methods Mol Biol. 2024. PMID: 39141235
-
More Than Meets the Kappa for Antibody Superantigen Protein L (PpL).Antibodies (Basel). 2022 Feb 11;11(1):14. doi: 10.3390/antib11010014. Antibodies (Basel). 2022. PMID: 35225872 Free PMC article.
-
Binding of Immunoglobulin G to Protoporphyrin IX and Its Derivatives: Evidence the Fab Domain Recognizes the Protoporphyrin Ring.Antibodies (Basel). 2019 Jan 4;8(1):6. doi: 10.3390/antib8010006. Antibodies (Basel). 2019. PMID: 31544812 Free PMC article.
References
-
- Steinitz M. Three decades of human monoclonal antibodies: Past, present and future developments. Hum. Antibodies. 2009;18:1–10. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

