The clinical utility of the urine-based lateral flow lipoarabinomannan assay in HIV-infected adults in Myanmar: an observational study
- PMID: 28774293
- PMCID: PMC5543584
- DOI: 10.1186/s12916-017-0888-3
The clinical utility of the urine-based lateral flow lipoarabinomannan assay in HIV-infected adults in Myanmar: an observational study
Abstract
Background: The use of the point-of-care lateral flow lipoarabinomannan (LF-LAM) test may expedite tuberculosis (TB) diagnosis in HIV-positive patients. However, the test's clinical utility is poorly defined outside sub-Saharan Africa.
Methods: The study enrolled consecutive HIV-positive adults at a tertiary referral hospital in Yangon, Myanmar. On enrolment, patients had a LF-LAM test performed according to the manufacturer's instructions. Clinicians managing the patients were unaware of the LF-LAM result, which was correlated with the patient's clinical course over the ensuing 6 months.
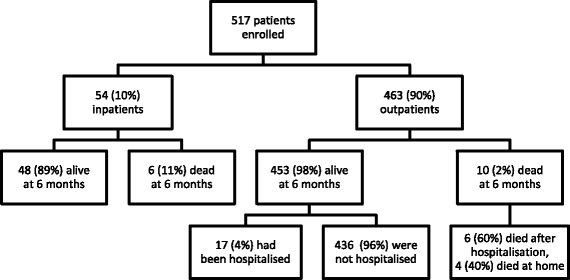
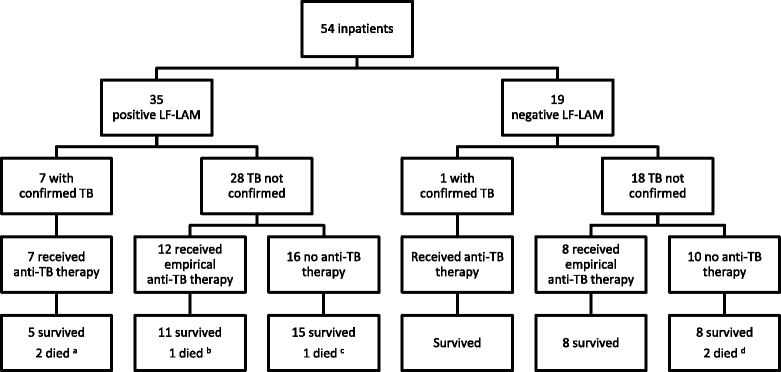
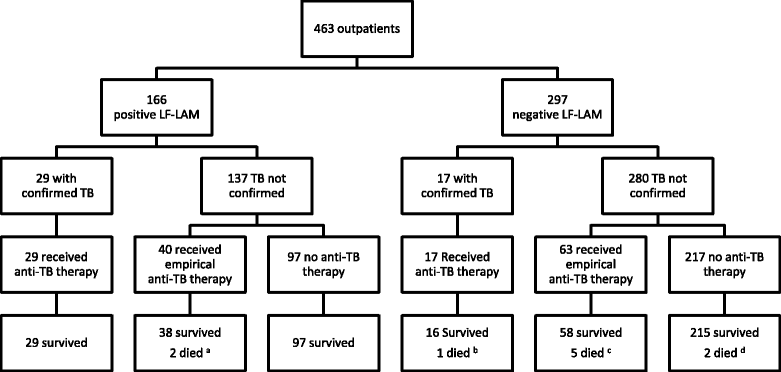
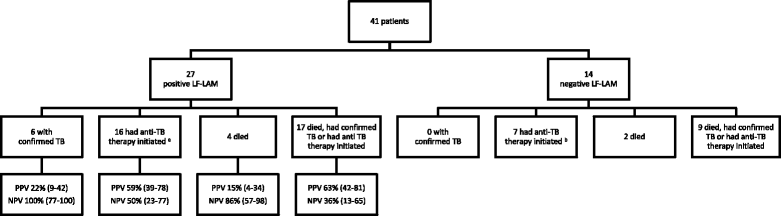
Results: The study enrolled 54 inpatients and 463 outpatients between July 1 and December 31, 2015. On enrolment, the patients' median (interquartile range) CD4 T-cell count was 270 (128-443) cells/mm3. The baseline LF-LAM test was positive in 201/517 (39%). TB was confirmed microbiologically during follow-up in 54/517 (10%), with rifampicin resistance present in 8/54 (15%). In the study's resource-limited setting, extrapulmonary testing for TB was not possible, but after 6 months, 97/201 (48%) with a positive LF-LAM test on enrolment had neither died, required hospitalisation, received a TB diagnosis or received empirical anti-TB therapy, suggesting a high rate of false-positive results. Of the 97 false-positive tests, 89 (92%) were grade 1 positive, suggesting poor test specificity using this cut-off. Only 21/517 (4%) patients were inpatients with TB symptoms and a CD4 T-cell count of < 100 cells/mm3. Five (24%) of these 21 died, three of whom had a positive LF-LAM test on enrolment. However, all three received anti-TB therapy before death - two after diagnosis with Xpert MTB/RIF testing, while the other received empirical treatment. It is unlikely that knowledge of the baseline LF-LAM result would have averted any of the study's other 11 deaths; eight had a negative test, and of the three patients with a positive test, two received anti-TB therapy before death, while one died from laboratory-confirmed cryptococcal meningitis. The test was no better than a simple, clinical history excluding TB during follow-up (negative predictive value (95% confidence interval): 94% (91-97) vs. 94% (91-96)).
Conclusions: The LF-LAM test had limited clinical utility in the management of HIV-positive patients in this Asian referral hospital setting.
Keywords: Clinical management; Diagnostic test; Human immunodeficiency virus; Lipoarabinomannan; Myanmar; Tuberculosis.
Figures
References
-
- World Health Organization . Global Tuberculosis Report 2016. Geneva: WHO; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

