What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis
- PMID: 28774325
- PMCID: PMC5543539
- DOI: 10.1186/s13054-017-1796-9
What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis
Abstract
Background: The fluid challenge is considered the gold standard for diagnosis of fluid responsiveness. The objective of this study was to describe the fluid challenge techniques reported in fluid responsiveness studies and to assess the difference in the proportion of 'responders,' (PR) depending on the type of fluid, volume, duration of infusion and timing of assessment.
Methods: Searches of MEDLINE and Embase were performed for studies using the fluid challenge as a test of cardiac preload with a description of the technique, a reported definition of fluid responsiveness and PR. The primary outcome was the mean PR, depending on volume of fluid, type of fluids, rate of infusion and time of assessment.
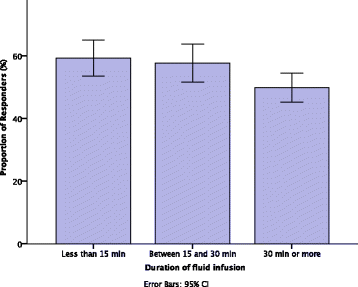
Results: A total of 85 studies (3601 patients) were included in the analysis. The PR were 54.4% (95% CI 46.9-62.7) where <500 ml was administered, 57.2% (95% CI 52.9-61.0) where 500 ml was administered and 60.5% (95% CI 35.9-79.2) where >500 ml was administered (p = 0.71). The PR was not affected by type of fluid. The PR was similar among patients administered a fluid challenge for <15 minutes (59.2%, 95% CI 54.2-64.1) and for 15-30 minutes (57.7%, 95% CI 52.4-62.4, p = 1). Where the infusion time was ≥30 minutes, there was a lower PR of 49.9% (95% CI 45.6-54, p = 0.04). Response was assessed at the end of fluid challenge, between 1 and 10 minutes, and >10 minutes after the fluid challenge. The proportions of responders were 53.9%, 57.7% and 52.3%, respectively (p = 0.47).
Conclusions: The PR decreases with a long infusion time. A standard technique for fluid challenge is desirable.
Keywords: Fluid challenge; Fluid responsiveness; Fluid resuscitation; Fluid therapy.
Conflict of interest statement
Ethics approval and consent to participate
No ethical approval or patient consent was necessary for the present study.
Consent for publication
Not applicable.
Competing interests
HDA received financial support from LiDCO for educational programs and for attending symposia. AR has received honoraria for serving on an advisory board for LiDCO, as well as honoraria from Covidien, Edwards Lifesciences and Cheetah. MC has received honoraria for speaking at symposia, financial support for educational programs and honoraria for serving on an advisory board from Edwards Lifesciences, LiDCO, Deltex, Massimo, BMEYE, Cheetah and ImaCor. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(25):2462–2463. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

