18F-FDG PET/CT response in a phase 1/2 trial of nab-paclitaxel plus gemcitabine for advanced pancreatic cancer
- PMID: 28774338
- PMCID: PMC5543580
- DOI: 10.1186/s40644-017-0125-5
18F-FDG PET/CT response in a phase 1/2 trial of nab-paclitaxel plus gemcitabine for advanced pancreatic cancer
Abstract
Background: Positron emission tomography (PET) is poised to become a useful imaging modality in staging and evaluating therapeutic responses in patients with metastatic pancreatic cancer (mPC). This analysis from a phase 1/2 study examined the utility of early PET imaging in patients with mPC treated with nab-paclitaxel plus gemcitabine.
Methods: Tumors were measured by [18F]2-fluoro-2-deoxyglucose PET/computed tomography (CT) in patients who received nab-paclitaxel 100 (n = 13), 125 (n = 38), or 150 (n = 1) mg/m2 plus gemcitabine 1000 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Lesion metabolic activity was evaluated at baseline and 6 and 12 weeks postbaseline.
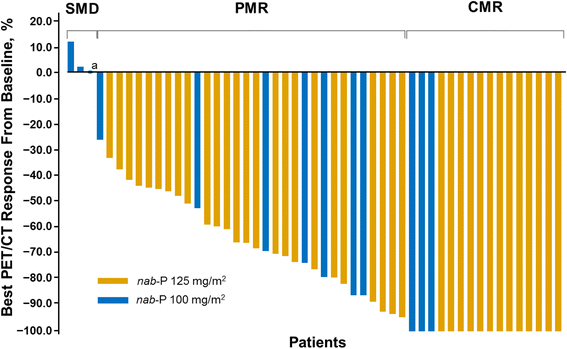
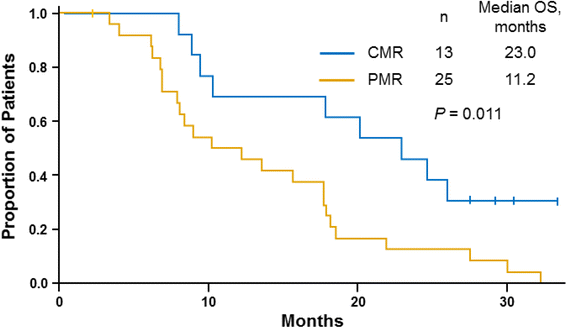
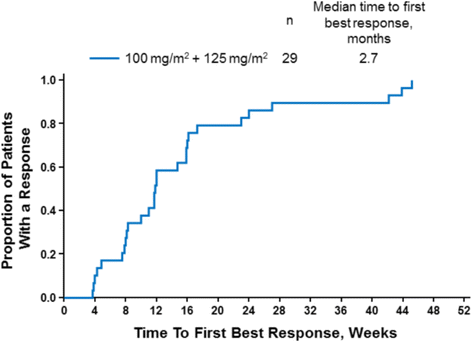
Results: Fifty-two patients had baseline and ≥1 follow-up PET scan. The median maximum standardized uptake values per pancreatic lesion in the nab-paclitaxel 100 mg/m2 and 125 mg/m2 cohorts were 5.1 and 6.5, respectively. Among patients who had a metabolic response by PET, those who received nab-paclitaxel 125 mg/m2 had a 4-month survival advantage over those who received 100 mg/m2. All patients in the nab-paclitaxel 125 mg/m2 cohort experienced an early complete metabolic response (CMR; 34%) or partial metabolic response (PMR; 66%). In the nab-paclitaxel 125 mg/m2 cohort, investigator-assessed objective response rates were 77% and 44% among patients with a CMR and PMR, respectively, with no correlation between PET and CT response (Spearman r s = 0.22; P = 0.193). Patients in the nab-paclitaxel 125 mg/m2 cohort with a CMR experienced a significantly longer overall survival vs those with a PMR (median, 23.0 vs 11.2 months; P = 0.011), and a significant correlation was found between best percentage change in tumor burden by PET and survival: for each 1% decrease in PET score, the risk of death decreased by 2%.
Conclusions: The majority of primary pancreatic tumors and their metastases were PET avid, and PET effectively measured changes in tumor metabolic activity at 6 and 12 weeks. These results support the antitumor activity of nab-paclitaxel 125 mg/m2 plus gemcitabine 1000 mg/m2 for treating mPC and the utility of PET for measuring treatment response. Treatment response by PET analysis may be considered when evaluating investigational agents in mPC.
Trial registration: NCT00398086.
Keywords: Gemcitabine; Pancreatic cancer; Phase 1/2 clinical trial; Positron emission tomography; nab-Paclitaxel.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, Guidelines of the International Conference on Harmonization. Written informed consent was obtained from all patients before entering the study.
Consent for publication
Not applicable.
Competing interests
RLK: research funding: Celgene; DDVH: consultant or advisory role, honoraria, and research funding: Celgene; MJB: nothing to disclose; RCB: consultant: Celgene; DM: employment: Celgene; MFR: employment: Celgene; RKR: consultant or advisory role, honoraria, and research funding: Celgene.
The manuscript has not been submitted for publication nor is it under consideration for publication elsewhere.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Positron emission tomography response evaluation from a randomized phase III trial of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas.Ann Oncol. 2016 Apr;27(4):648-53. doi: 10.1093/annonc/mdw020. Epub 2016 Jan 22. Ann Oncol. 2016. PMID: 26802153 Free PMC article. Clinical Trial.
-
Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial.J Clin Oncol. 2011 Dec 1;29(34):4548-54. doi: 10.1200/JCO.2011.36.5742. Epub 2011 Oct 3. J Clin Oncol. 2011. PMID: 21969517 Free PMC article. Clinical Trial.
-
Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study.Lancet Gastroenterol Hepatol. 2020 Mar;5(3):285-294. doi: 10.1016/S2468-1253(19)30327-9. Epub 2020 Jan 14. Lancet Gastroenterol Hepatol. 2020. PMID: 31953079 Clinical Trial.
-
Nab-paclitaxel and gemcitabine for the treatment of patients with metastatic pancreatic cancer.Expert Rev Gastroenterol Hepatol. 2014 Sep;8(7):739-47. doi: 10.1586/17474124.2014.925799. Epub 2014 May 31. Expert Rev Gastroenterol Hepatol. 2014. PMID: 24882381 Review.
-
Treatment of metastatic pancreatic adenocarcinoma: a review.Oncology (Williston Park). 2014 Jan;28(1):70-4. Oncology (Williston Park). 2014. PMID: 24683721 Review.
Cited by
-
Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review.Diagnostics (Basel). 2020 Dec 3;10(12):1042. doi: 10.3390/diagnostics10121042. Diagnostics (Basel). 2020. PMID: 33287195 Free PMC article. Review.
References
-
- Qiao W, Zhao J, Xing Y, Wang C, Wang T. Predictive value of [18F]fluoro-2-deoxy-d-glucose positron emission tomography for clinical outcome in patients with relapsed/refractory diffuse large B-cell lymphoma prior to and after autologous stem cell transplant. Leuk Lymphoma. 2014;55:276–282. doi: 10.3109/10428194.2013.797974. - DOI - PubMed
-
- Gebhart G, Gamez C, Holmes E, Robles J, Garcia C, Cortes M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from neo-ALTTO. J Nucl Med. 2013;54:1862–1868. doi: 10.2967/jnumed.112.119271. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

