Management of hepatitis B in special populations
- PMID: 28774413
- PMCID: PMC6548717
- DOI: 10.1016/j.bpg.2017.06.002
Management of hepatitis B in special populations
Abstract
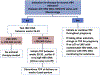
Special populations infected with chronic HBV include those with decompensated cirrhosis, coinfections (HIV, HCV, HDV), hemodialysis and renal failure, immunosuppressed including transplant patients, children and women in pregnancy. These populations differ in their natural history and risk for liver-related complications, the indications for anti-HBV therapy as well as the recommendations regarding the HBV drugs used, duration of therapy and anticipated endpoints. Reflecting the special populations with substantive changes in management in recent years, this review focuses on HBV-HIV coinfected patients, immunosuppressed patients at risk for reactivation, liver transplant recipients and pregnant women. Management of women in the context of pregnancy and post-partum requires consideration of risks to mother and fetus/infant, including the risk of mother-to-child transmission. HBV-HIV coinfected patients require initiation of treatment concurrent with their HIV therapy and the HBV drugs used must by selected to minimize HIV and HBV resistance long-term. Increasing recognition of the risk for HBV reactivation with immunosuppressive therapy has led to recommendations to use prophylactic HBV therapy in patients with moderate to high risk of reactivation. Liver transplant recipients with HBV require life-long therapy to prevent or treat HBV infection but with current therapies, graft and patient survival are excellent.
Keywords: Entecavir; HBV–HIV coinfection; Nucleos(t)ide analogues; Pregnancy; Reactivation; Tenofovir; Transplantation.
Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest statement
KZ reports grant support from T32 DK060414. NT reports grant support from Gilead Sciences and BMS.
Figures

References
-
- Beasley RP, Hwang LY, Stevens CE, Lin CC, Hsieh FJ, Wang KY, et al. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology 1983;3(2):135–41. - PubMed
-
- Yi W, Pan CQ, Hao J, Hu Y, Liu M, Li L, et al. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J Hepatol 2014;60(3):523–9. - PubMed
-
- Wiseman E, Fraser M, Holden S, Glass A, Kidson B, Heron L, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust 2009;190(9):489–92. - PubMed
-
- WHO. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection May 12, 2015. - PubMed
-
- Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut 2015;64(11):1810–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

