Correlation between anthrax lethal toxin neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate
- PMID: 28774566
- PMCID: PMC5580334
- DOI: 10.1016/j.vaccine.2017.07.076
Correlation between anthrax lethal toxin neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate
Abstract
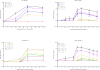
The anthrax vaccine candidate AV7909 is being developed as a next generation vaccine for a post-exposure prophylaxis (PEP) indication against anthrax. AV7909 consists of the Anthrax Vaccine Adsorbed (AVA, BioThrax®) bulk drug substance adjuvanted with the immunostimulatory oligodeoxynucleotide (ODN) compound, CPG 7909. The addition of CPG 7909 to AVA enhances both the magnitude and the kinetics of antibody responses in animals and human subjects, making AV7909 a suitable next-generation vaccine for use in a PEP setting. The studies described here provide initial information on AV7909-induced toxin-neutralizing antibody (TNA) levels associated with the protection of animals from lethal Bacillus anthracis challenge. Guinea pigs or nonhuman primates (NHPs) were immunized on Days 0 and 28 with various dilutions of AV7909, AVA or a saline or Alhydrogel+CPG 7909 control. Animals were challenged via the inhalational route with a lethal dose of aerosolized B. anthracis (Ames strain) spores and observed for clinical signs of disease and mortality. The relationship between pre-challenge serum TNA levels and survival following challenge was determined in order to calculate a threshold TNA level associated with protection. Immunisation with AV7909 induced a rapid, highly protective TNA response in guinea pigs and NHPs. Surprisingly, the TNA threshold associated with a 70% probability of survival for AV7909 immunized animals was substantially lower than the threshold which has been established for the licensed AVA vaccine. The results of this study suggest that the TNA threshold of protection against anthrax could be modified by the addition of an immune stimulant such as CPG 7909 and that the TNA levels associated with protection may be vaccine-specific.
Keywords: Animal rule; Anthrax vaccine; CPG 7909 adjuvant; Correlate of protection; Cynomolgus macaque; Guinea pig; TNA threshold.
Copyright © 2017. Published by Elsevier Ltd.
Figures
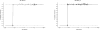
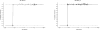
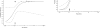
References
-
- Longstreth J, Skiadopoulos MH, Hopkins RJ. Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: the first vaccine licensed using the FDA animal rule. Expert review of vaccines. 2016;15(12):1467–79. - PubMed
-
- Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

