Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study
- PMID: 28774879
- PMCID: PMC5649550
- DOI: 10.1182/blood-2017-03-769620
Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study
Erratum in
-
Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808.Blood. 2018 Feb 1;131(5):587-588. doi: 10.1182/blood-2017-11-817775. Blood. 2018. PMID: 29437609 Free PMC article. No abstract available.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma. Although 5-year survival rates in the first-line setting range from 60% to 70%, up to 50% of patients become refractory to or relapse after treatment. Published analyses of large-scale outcome data from patients with refractory DLBCL are limited. SCHOLAR-1, an international, multicohort retrospective non-Hodgkin lymphoma research study, retrospectively evaluated outcomes in patients with refractory DLBCL which, for this study, was defined as progressive disease or stable disease as best response at any point during chemotherapy (>4 cycles of first-line or 2 cycles of later-line therapy) or relapsed at ≤12 months from autologous stem cell transplantation. SCHOLAR-1 pooled data from 2 phase 3 clinical trials (Lymphoma Academic Research Organization-CORAL and Canadian Cancer Trials Group LY.12) and 2 observational cohorts (MD Anderson Cancer Center and University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence). Response rates and overall survival were estimated from the time of initiation of salvage therapy for refractory disease. Among 861 patients, 636 were included on the basis of refractory disease inclusion criteria. For patients with refractory DLBCL, the objective response rate was 26% (complete response rate, 7%) to the next line of therapy, and the median overall survival was 6.3 months. Twenty percent of patients were alive at 2 years. Outcomes were consistently poor across patient subgroups and study cohorts. SCHOLAR-1 is the largest patient-level pooled retrospective analysis to characterize response rates and survival for a population of patients with refractory DLBCL.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: S.S.N. received research funding from and served as a consultant and an advisory board member for Kite Pharma. U.F. received research funding from Kite Pharma. J.K. received research funding from Celgene, Roche, and Karyopharm; served as a consultant for Bristol-Myers Squibb, Gilead, Janssen, Hoffman LaRoche, and Seattle Genetics; and received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Gilead, Roche, Janssen, Lundbeck, Merck, and Seattle Genetics. J.W. served on advisory boards for ProNAi Therapeutics, Celgene, and Genentech and received research funding from Janssen, Celgene, Genentech, Kite, and Novartis. B.K.L., J.R.C., and M.J.M. received research funding from Kite Pharma. L.N., J.W., and W.Y.G. are employed by and have equity ownership in Kite Pharma. The remaining authors declare no competing financial interests.
Figures
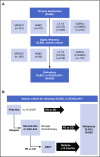
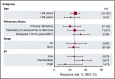
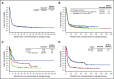
Comment in
-
Lymphoma "benchmark" or "bench-smudge"?Blood. 2017 Oct 19;130(16):1778-1779. doi: 10.1182/blood-2017-08-800730. Blood. 2017. PMID: 29051150 No abstract available.
References
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes [published online ahead of print 12 September 2016]. CA Cancer J Clin. doi: 10.3322/caac.21357. - PubMed
-
- Tilly H, Vitolo U, Walewski J, et al. ; ESMO Guidelines Working Group. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii78-vii82. - PubMed
-
- Howlader N, Noon AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute, Bethesda, MD, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. https://seer.cancer.gov/csr/1975_2013/.
-
- Sant M, Minicozzi P, Mounier M, et al. ; EUROCARE-5 Working Group. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15(9):931-942. - PubMed
-
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498-505. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

